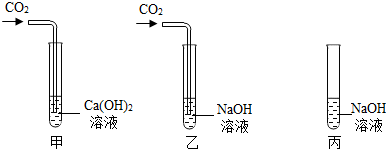
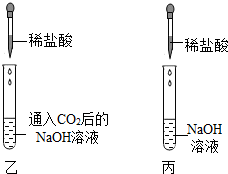
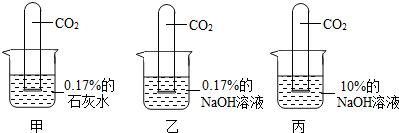
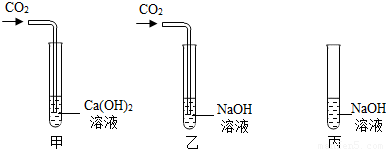
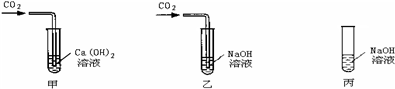
17������ͬѧ��ѧϰ�������ļʱ������������⣺
�ټ�������������̼Ϊʲôһ���ó���ʯ��ˮ������NaOH��Һ��
��ʵ��ʱ���ս϶��CO
2Ϊʲôһ����NaOH��Һ�����ó���ʯ��ˮ��
[��������]a.20��ʱ����ʯ�ҵ��ܽ��Ϊ0.17g��b����ʯ�ҵ��ܽ�������¶����߶����ͣ�
[ʵ��̽��]�ڽ�ʦָ���£�����ͬѧ����������ʵ��̽����
̽��һ�����ڶ�����̼�ļ���
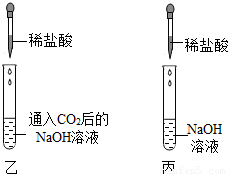
���ڼס��ҡ�����֧�Թ��м������NaOH��Ca��OH��
2 ��Һ������ͼ�����ڼס����зֱ�ͨ������CO
2��
����ʵ�����Һͱ����ֳ�����Һ�У��ֱ��������ϡ���ᣨ����ͼ����

�ش����⣺
��1�����мס��Ҷ�֧�Թܵ�����ֱ���
���Թܲ�����ɫ�����������ǣ������Թ�����������
��
��2���������Թܿɹ۲쵽�����ݲ�����д���÷�Ӧ�Ļ�ѧ����ʽ��
Na2CO3+2HCl�T2NaCl+CO2��+H2O
����Ʊ��Թ�ʵ���Ŀ����
�������ԹܵĶԱ�ʵ�飬֤�����Թ���CO2��NaOH�����˻�ѧ��Ӧ
��
[����]��������CO
2һ����ʯ��ˮ������NaOH��Һ��
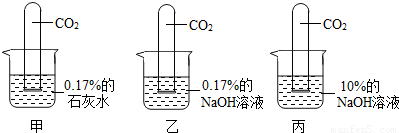
̽���������ڶ�����̼������
��������ͼ��ʾʵ�飺
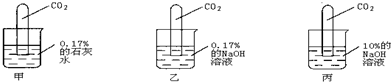
[ʵ������]���Թܣ�Һ���������٣� ���Թܣ�Һ�������ϼ��Ըߣ�
���Թܣ������ӽ���ȫ�����ڹܵ�����һС���ݣ�
��3��С����Ϊ����ֱ������10%��ʯ��ˮ�������0.17%��ʯ��ˮ��������жԱ�ʵ�飮��ͬ��С�յĹ۵��𣿴�
��ͬ��
���ͬ�⡱��ͬ�⡱����������
20��ʱ����ʯ�ҵ��ܽ����0.17g�������Ƴ�10%��ʯ��ˮ����20��ʱ0.17%��ʯ��ˮ�DZ�����Һ��ʯ��ˮ�������������������ܴﵽ10%������������Ҳ���֣�
��4���Ƚ��ҡ����н����Թ���Һ��������ó��Ľ�����
NaOH��Һ��������������Խ������CO2����Խǿ
��
[�ó�����]ʵ��ʱ����CO
2ѡ�ý�Ũ��NaOH��Һ������Ca��OH��
2��Һ��������
����ʱҪ��������CO2��NaOH��������ˮ���γɵ���ҺŨ�ȴ��������࣬Ч��ߣ���Ca��OH��2�ܣ�����������CO2Ҫ�������Һ
��
[ʵ����չ]Ϊ��ֱ�۵ع۲쵽CO
2��NaOH��Ӧ���������������ͼװ�ý���ʵ�飮����NaOH��Һ�ĵ��룬�ɹ۲쵽��������
С�������ʹ�
��