
С�մ�(��Ҫ�ɷ�ΪNaHCO3)�г����������Ȼ��ơ���ѧ��ȤС���ͬѧΪ�˲ⶨijƷ��С�մ���NaHCO3����������������������ʵ�飺������Ʒ�����ձ��У������������μ�ϡ���ᣬ�����ٲ�������Ϊֹ����õ��й��������±���ʾ��
���� | ��Ʒ | ���ĵ�ϡ���� | ��Ӧ�����Һ |
����(g) | 9 | 75.4 | 80 |
�Լ���(����������һλС��)��
(1)��Ʒ�е�NaHCO3������������
(2)������Һ��NaCl������������
��1��93.3%?????? ��2��8.1%
���������÷�Ӧ�Ļ�ѧ����ʽΪNaHCO3+HCl=NaCl+H2O+CO2��,��Ӧ�����ɵĶ�����̼���������Ϊ9 g+75.4 g-80 g=4.4 g,��Ʒ�е�NaHCO3��������������Һ��NaCl������������CO2�����������
�⣺��Ӧ�����ɵĶ�����̼���������Ϊ9 g+75.4 g-80 g=4.4 g��
����Ʒ�е�NaHCO3����Ϊx�����ɵ�NaCl������Ϊy
NaHCO3+HCl=NaCl+H2O+CO2��
? 84???????? 58.5???? 44
?? x????????? y????? 4.4 g
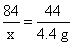 ? x=8.4 g
? x=8.4 g
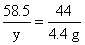 ?? y=5.85 g
?? y=5.85 g
(1)��Ʒ�е�NaHCO3����������Ϊ��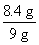 ��100%��93.3%
��100%��93.3%
(2)��Ʒ��NaCl������=9 g -8.4 g=0.6 g����Һ��NaCl��������=0.6 g+5.85 g=6.45 g��������Һ��NaCl����������Ϊ��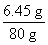 ��100%��8.1%
��100%��8.1%



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� �� | �� Ʒ | ����ϡ�������� | ��Ӧ����Һ���� |
| ������g�� | 9 | 75.4 | 80 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)С�մ�(��Ҫ�ɷ�ΪNaHC03)�г����������Ȼ��ơ�С��ͬѧΪ�˲ⶨijƷ��С�մ���NaHC03�������������������ϣ�NaHC03+HCl==NaCl+H20+C02����Ȼ�����������ʵ�飺������Ʒ�����ձ��У������������μ�ϡ���ᣬ�����ٲ�������Ϊֹ������й��������±���ʾ���Լ��㣺(����������һλС��)
| ���� | ��Ʒ | ����ϡ�������� | ��Ӧ����Һ���� |
| ����(g) | 9 | 75.4 | 80 |
(1)���ɵĶ�����̼������Ϊ________��
(2)��Ʒ�е�NaHC03����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�����о��꼶��һѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�������
(6��)С�մ�(��Ҫ�ɷ�ΪNaHC03)�г����������Ȼ��ơ�С��ͬѧΪ�˲ⶨijƷ��С�մ���NaHC03�������������������ϣ�NaHC03+HCl==NaCl+H20+C02����Ȼ�����������ʵ�飺������Ʒ�����ձ��У������������μ�ϡ���ᣬ�����ٲ�������Ϊֹ������й��������±���ʾ���Լ��㣺(����������һλС��)
|
���� |
��Ʒ |
����ϡ�������� |
��Ӧ����Һ���� |
|
����(g) |
9 |
75.4 |
80 |
(1)���ɵĶ�����̼������Ϊ________��
(2)��Ʒ�е�NaHC03����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С�մ�(��Ҫ�ɷ�ΪNaHCO3)�г����������Ȼ��ơ���ѧ��ȤС���ͬѧΪ�˲ⶨijƷ��С�մ���NaHCO3����������������������ʵ�飺������Ʒ�����ձ��У������������μ�ϡ���ᣬ�����ٲ�������Ϊֹ����õ��й��������±���ʾ��
| ���� | ��Ʒ | ����ϡ�������� | ��Ӧ����Һ���� |
| ����/g | 9 | 75.4 | 80 |
�Լ��㣺(����������һλС��)
(1)��Ʒ�е�NaHCO3����������
(2)������Һ��NaCl������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com