
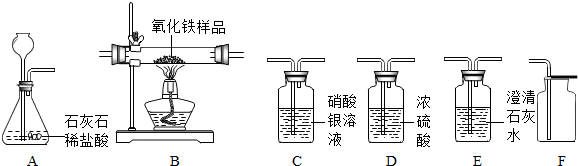
ij��ѧ��ȤС����ʵ�����ö������̷�ĩ����������Һ��O2���������������������������ݸ�С���ʵ����̣������Ӧ��ա�
��С���һ��ʵ��ʱ��������ͼһ��ʾ��װ����ȡ�������仯ѧʽ����ʽΪ��
����ʵ��ʱ�������������⣺


����һ��ʵ��ʱ�Թ����кܶ���ĭ����Щ��ĭ���������˵��ܡ���С���ڲ�������������������£��Ը�װ������һ���ĸĽ�����Ч����ֹ����ĭ���뵼�ܣ�����ܵ������ǣ�
��
�������ʵ��ʱ���������ٶȹ��죬�ռ�һ��������ʱҩƷ�˷ѽ϶ࡣ��С���������ͼ����ʾװ����ȡ�����������������ĭ���뵼�ܵ����⣬���ҽϺõؿ����˷�Ӧ�ٶȡ���װ���ܽϺÿ��Ʒ�Ӧ�ٶ�����Ϊ��_______________
____
��С������ͼ��װ����ȡ����ʱ�ַ����������⣬������ʱ��Һ�岻��˳���ص�����ƿ�У��෴��ʱ�������ݴӷ���©����������������ԭ���ǣ�
��Ϊ�˽��������⣬��ʦ�����С��Ը�װ������һ���ĸĽ�����һ���µ����������ϵ��ܣ�һͷ������ƿ����Ƥ���ϣ�һͷ������Ӧ����Ƥ����������Ƥ�����ڷ�Һ©�����Ͽڡ�����ʵ�飬���ָĽ����ʵ��Ƚ�˳����
����ͼ��װ����ͼ��װ��������һ�����ø�װ�ò�÷�Ӧ�����������ƫ��������Ҫԭ���ǣ�
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
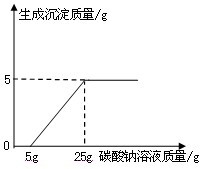 ij��ѧ��ȤС������ʯ��ʯ�����ʲ����ᷴӦ��Ҳ������ˮ����ϡ���ᷴӦ��ȡ������̼����������Ӧ��ķ�Һ������Һ��ʱ������ʵ��������һƿδ֪����������Na2CO3��Һ�����Ǿ������ø÷�Һ�ⶨ��ƿNa2CO3��Һ���������������������Ƚ���Һ���ˣ�Ȼ�����Һ�������μ�Na2CO3��Һ������Na2CO3��Һ�����������ɳ��������Ĺ�ϵ����ͼ��ʾ��
ij��ѧ��ȤС������ʯ��ʯ�����ʲ����ᷴӦ��Ҳ������ˮ����ϡ���ᷴӦ��ȡ������̼����������Ӧ��ķ�Һ������Һ��ʱ������ʵ��������һƿδ֪����������Na2CO3��Һ�����Ǿ������ø÷�Һ�ⶨ��ƿNa2CO3��Һ���������������������Ƚ���Һ���ˣ�Ȼ�����Һ�������μ�Na2CO3��Һ������Na2CO3��Һ�����������ɳ��������Ĺ�ϵ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
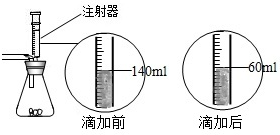 ij��ѧ��ȤС����ʵ�������ȡ������̼����ѡ������ͼ�ķ���װ�ã�����ȡ��Ϻ���ͬѧ�����װ�û��ܲ��ϡ���������������С��ͬѧ��ȡ12.5g��CaCO380%��ʯ��ʯ��ĩ��Ʒ����װ���У���ע�����μ�ϡ������ǡ����ȫ��Ӧ���μ�ǰ�����������ͼ��ʾ����������ϡ�����Ӧ��ϡ������ܶ�Ϊ1.25g/mL��
ij��ѧ��ȤС����ʵ�������ȡ������̼����ѡ������ͼ�ķ���װ�ã�����ȡ��Ϻ���ͬѧ�����װ�û��ܲ��ϡ���������������С��ͬѧ��ȡ12.5g��CaCO380%��ʯ��ʯ��ĩ��Ʒ����װ���У���ע�����μ�ϡ������ǡ����ȫ��Ӧ���μ�ǰ�����������ͼ��ʾ����������ϡ�����Ӧ��ϡ������ܶ�Ϊ1.25g/mL���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
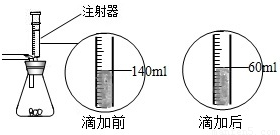
 ij��ѧ��ȤС����ʵ�������ȡ������̼����ѡ������ͼ�ķ���װ�ã�����ȡ��Ϻ���ͬѧ�����װ�û��ܲ��ϡ���������������С��ͬѧ��ȡ12.5g��CaCO380%��ʯ��ʯ��ĩ��Ʒ����װ���У���ע�����μ�ϡ������ǡ����ȫ��Ӧ���μ�ǰ�����������ͼ��ʾ����������ϡ�����Ӧ��ϡ������ܶ�Ϊ1.25g/mL��
ij��ѧ��ȤС����ʵ�������ȡ������̼����ѡ������ͼ�ķ���װ�ã�����ȡ��Ϻ���ͬѧ�����װ�û��ܲ��ϡ���������������С��ͬѧ��ȡ12.5g��CaCO380%��ʯ��ʯ��ĩ��Ʒ����װ���У���ע�����μ�ϡ������ǡ����ȫ��Ӧ���μ�ǰ�����������ͼ��ʾ����������ϡ�����Ӧ��ϡ������ܶ�Ϊ1.25g/mL���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��������������������ѧ�п���ѧ��ģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com