�ߺ���������300����ķ�չ���ѳ�Ϊһ����Է���⣬����Ϊ�����������Ļ��Ų�����������������ʦ���ָ������µ�������������������һ����ɫ������--�ƽ�
��1������������ƽ��������______��______��
��2����֪�ƽ�������ֻ���н�����̼����Ԫ�أ�ijͬѧΪ̽���ƽ�����������Ԫ�صĺ���������������ʵ�飺ȡ�ƽ������ı߽���6g��ĥ�ɷ�ĩ������������ϡ�����У��ռ���0.2g��������Һ���˺�����ϴ������ɣ��ڿ����г�����գ�ʣ����������Ϊ0.24g���������н�����̼������������
���𰸡�
��������1����֪����������ƽ�����ã���֪�������������������ʣ�
��2����Ϊ������̼������Ԫ�أ�ֻ�������Ժ����ᷢ����Ӧ�������������ʿɸ��ݻ�ѧ����ʽ�ó���Ӧ��֮��ı���ʽ����������������������֪����Һ���˺�����ϴ������ɣ��ڿ����г�����գ�ʣ����������Ϊ0.24g����Ϊ���Ӳ�ȸߣ������ȶ�������ʣ�������ǽ�̼���������DZ߽��ϵ�����-��������-���������Ȼ���������������ʽ���������н�����̼������������
����⣺��1����������Ҫ����������������������ǣ���ͬʱֱ�Ӻ�������ˮ�Ӵ�������������¶�ڿ����У��߱������Ӵ��������������в�����ˮ�֣������쳤�վã����������⣮�������ƽ���ֹ��������������ˮ�ĽӴ������Է�ֹ�������⣬ʹ�����ܳ�ʱ�䱣�森ͬʱ�����ɫ�������Ƚ����ۣ��������������ƽ�������Ƿ�ֹ���⡢���ۣ�
��2����Ϊ������̼������Ԫ�أ�ֻ�������Ժ����ᷢ����Ӧ�������������ʿɸ��ݻ�ѧ����ʽ�ó���Ӧ��֮��ı���ʽ���������������
�⣺��6.0g�����߽�����������������Ϊx��
Fe+2HCl=FeCl
2+H
2��
56 2
x 0.2g

��֮��x=5.6g
��������������
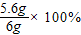
=93.33%
��Ϊ���Ӳ�ȸߣ������ȶ�������ʣ�������ǽ𣬽����������Ϊ��
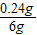
×100%=4%
̼���������DZ߽��ϵ�����-��������-�����������̼����������Ϊ��

×100%=2.67%
�������н�����̼�����������ֱ�Ϊ4%��93.33%��2.67%��
������������Ҫ����ѧ������������������ʶ���Լ����������������ļ���������ѧ��������֪�������ʵ����ʣ�������ȷ����������Ӧ�Ĺ��̣��ݴ��м��������������������������������


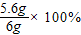 =93.33%
=93.33%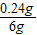 ×100%=4%
×100%=4% ×100%=2.67%
×100%=2.67%

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�