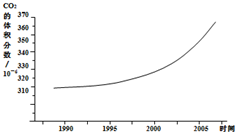
��ͼ��ij�п�������������������۲��ͳ�Ʒ��������ƵĶ�����̼�ڿ����еĺ����仯����ͼ��
��1����ͼ�пɿ���������̼����������
����
����
��������������ԭ���ǣ�
����ʹ�û�ʯȼ�ϣ������˴����Ķ�����̼
����ʹ�û�ʯȼ�ϣ������˴����Ķ�����̼
��
��2������Ϊ��ÿ���ļ��ж�����̼�ĺ�����ߵ���
��
��
����
��3��ľ̿�ڿ�������������ȼ�յĻ�ѧ����ʽΪ��
���˷���ʽ���������ڶ���ʹ��ȼ��ľ̿ȡůʱҪע��������ǣ�
ͨ��
ͨ��
��
��4��Ϊ�˽��Ͷ�����̼�Ի�����Ӱ�죬�������뽫�����еĶ�����̼ѹ����У��������ں�ˮ����ˮ������Ӧ��д��������̼��ˮ��Ӧ�Ļ�ѧ����ʽ��
CO2+H2O�TH2CO3
CO2+H2O�TH2CO3
��
��5���ҹ���ѧ�ҳ�Ǭ���������о��ɹ���������440���800����ѹ�����£�������̼�ͽ����Ʒ�Ӧ�������ʯ��̼���ƣ��ϳɵĽ��ʯ����1.2mm����ȫ�������㹤ҵ��;��
��1��������̼������Ʒ�Ӧ���ɽ��ʯ��̼���ƵĻ�ѧ����ʽΪ��
��
��2�������������ĽǶȷ��������ʯ��ˮ���˭������
ˮ������
ˮ������
�������ǣ�
����Ϊ����ˮ��ά����������������ʣ�û��ˮ��û��������û��ˮ��һ�����ォ������
����Ϊ����ˮ��ά����������������ʣ�û��ˮ��û��������û��ˮ��һ�����ォ������
��

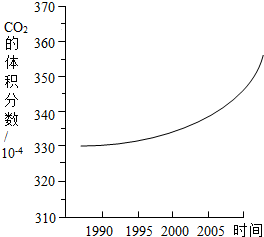 ��ͼ��ij�п�������������������۲�Ͷ����ռ�����ʷ���ϵ�ͳ���Ʒ��������ƵĶ�����̼�ڿ����еĺ����仯����ͼ��
��ͼ��ij�п�������������������۲�Ͷ����ռ�����ʷ���ϵ�ͳ���Ʒ��������ƵĶ�����̼�ڿ����еĺ����仯����ͼ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
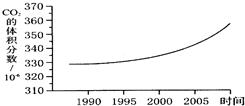
 ��ͼ��ij�п�������������������۲��ͳ�Ʒ��������ƵĶ�����̼�ڿ����еĺ����仯����ͼ��
��ͼ��ij�п�������������������۲��ͳ�Ʒ��������ƵĶ�����̼�ڿ����еĺ����仯����ͼ��
 ��ͼ��ij�п�������������������۲�Ͷ����ռ�����ʷ���ϵ�ͳ �Ʒ��������ƵĶ�����̼�ڿ����еĺ����仯����ͼ��
��ͼ��ij�п�������������������۲�Ͷ����ռ�����ʷ���ϵ�ͳ �Ʒ��������ƵĶ�����̼�ڿ����еĺ����仯����ͼ��