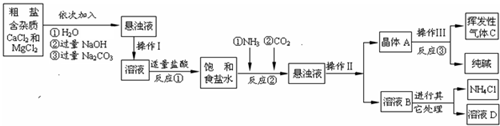
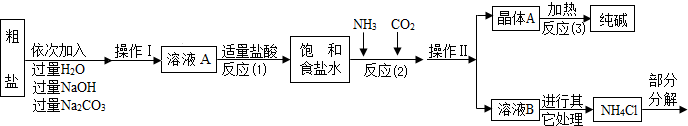
ijУ��ѧ��ȤС��ι��Ƽ���������Ϣ���������������о���
��Ʒԭ�����ó����á������Ƽ������������Ʒ--���Na
2CO
3���ͻ���NH
4Cl��
����ԭ���ǣ���NH
3��CO
2ͨ�뱥��ʳ��ˮ�еõ�NaHCO
3�����NH
4Cl��Һ����Ӧ�Ļ�ѧ����ʽΪ��______��������NaHCO
3�������Ƶô��
�������̣�

������ϣ�
��1��NH
4Cl

NH
3��+HCl��
��2����֪20��ʱ�й����ʵ��ܽ�����£�����ָ1���ˮ�����ܽ�����������
| ���� | NaCl | NaHCO3 | NH4Cl | NH3 | CO2 |
| �ܽ�� | 36.0g | 9.6g | 37.2g | 710 | 0.9 |
�������ۣ�
��1���������У���ͬ����������Ϊ______����Ӧ���з�����������Ӧ��д������һ����ѧ����ʽ��______����Ӧ�ټ����������ᣬ������ָ______��
��2������ӷ�������C�ķ���______��
��3���������������п�ѭ��ʹ�õ���______������ţ���
A���ӷ�������C�� B����ҺD�� C��������þ��D������NH
4Cl
���ȷ����
��1����ȡһ�������Ĵ�����Ʒ������γ�ּ��Ⱥ��ٳ��أ������ޱ仯��
��2����ȡ����������Ʒ��������ˮ����Ʒ��ȫ�ܽ⣬�����Һ�м������ϡHNO
3���ٵμ�AgNO
3��Һ���а�ɫ������������ʵ���ȷ��������Ʒ��������______��д��ѧʽ����
�����ⶨ��
| ʵ��һ | ʵ��� | ʵ���� | ʵ���� |
| ����������Һ���� | 100g | 100g | 100g | 100g |
| ����CaCl2��Һ���� | 10g | 20g | 30g | 40g |
| ���ɵij��������� | 4g | m | 10g | 10g |
��ȡ�ô�����Ʒ44g���������ˮ���400g��Һ��ƽ����Ϊ�ķݣ�Ȼ��ֱ����һ������������CaCl
2��Һ��ʵ�����ݼ�����
������������ݻش��㣺
��1��m=______g��
��2����ʵ�����У���ȫ��Ӧ��������Һ�������Ȼ��Ƶ����������Ƕ��٣���Ҫ��д������̣��������0.1%��
�ܽᷴ˼��
��ͨ�����㲢����±��жϴ˴�����Ʒ�ȼ�Ϊ______Ʒ��������������ⶨ�У���CaCl
2��Һ��ΪBaCl
2��Һ���ⶨ�����С��
| ��ҵ���������Na2CO3% �� |
| �ŵ�Ʒ | һ��Ʒ | �ϸ�Ʒ | ��Ʒ |
| ��95 | ��80 | ��75 | ��40 |


 NH3��+HCl��
NH3��+HCl��

 ��96.4%��95%���ʸô�����ƷΪ�ŵȣ�����ŵȣ�
��96.4%��95%���ʸô�����ƷΪ�ŵȣ�����ŵȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
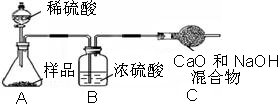 С�������������������ʸ����ȴ�����º�Ƶù�������Ϊ13.1g����Ʒ��̼���Ƶ���������Ϊ
С�������������������ʸ����ȴ�����º�Ƶù�������Ϊ13.1g����Ʒ��̼���Ƶ���������Ϊ