
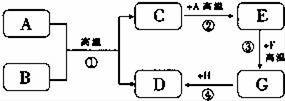
A��H���dz��л�ѧ�г��������ʣ���֪BΪ��ɫ���壬DΪ��ɫ���嵥�ʣ�FΪ��ɫ���壬���ǵ�ת����ϵ��ͼ��ʾ����ش�
��1������B�Ļ�ѧʽΪ������F�Ļ�ѧʽΪ������
��2��д�����з�Ӧ����ʽ
��Ӧ�ٵĻ�ѧ����ʽΪ����
��Ӧ�ڵĻ�ѧ����ʽΪ������
��3����Ӧ����������Ӧ���Ӧ�������ͣ�����Ӧ�١��۵ķ�Ӧ���������Ƿ���ͬ��������
Ϊ�˷�ֹʳƷ���ܣ�������ʳƷ��װ���ж���һ����ɫ��ĩ�����ַ�ĩ��һ�ֳ��������÷�ĩû��ʧЧʱ�ʺ�ɫ��ʧЧ����к���ɫ��Ϊ��ȷ���÷�ĩ�ijɷ֣�С�����������µġ����롿
����1���÷�ĩ��ľ̿��
����2���÷�ĩ������
����3���÷�ĩ��ľ̿�ۺ����۵Ļ����
С��ȡû��ʧЧ�ĸ÷�ĩ���ֱ��ò�ͬ�ķ�����������ʵ�飬��д���пո�
������� �������� �����Ƿ����
ʵ��1 ��������������ͭ��Һ ���� ����1��������
ʵ��2 ����������ϡ���� ������2������
ʵ��3 �ô������� ��ĩ��ȫ������ ����3������������
�������뷴˼��
��1������ʧЧ�����ɫ�жϣ��÷�ĩʧЧ��ԭ�������������ʺ�õ��ĺ���ɫ��ĩ����Ҫ�ɷ��������������ʵĻ�ѧʽ����
��2������ʲôʵ�鷽������ʹʧЧ�ķ�ĩ�������������û�ѧ����ʽ��ʾ��������

�����㡿���ʵļ����ƶϣ�ʵ��̽�����ʵ���ɳɷ��Լ������������Ļ�ѧ���ʣ���Ӧ���͵��ж�����д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ��
��ר�⡿��ͼ���ƶ��⣻���ʵļ��顢�������ƶϣ���ѧ̽����
������������BΪ��ɫ���壬DΪ��ɫ���嵥�ʣ���B����Ϊ����ͭ��D����Ϊͭ��A���л�ԭ�ԣ�������ͭ��Ӧ������C�ܹ���A��Ӧ����E�����A��̼�����ɵ�CΪ������̼��������̼����̼��Ӧ����һ����̼��EΪһ����̼��FΪ��ɫ���壬����һ����̼��Ӧ����FΪ�����������ɵ�GΪ����������ͭ�η�Ӧ����ͭ��H��������ͭ�������ͼ���ƶϺ�����
���ݲ����֪����ɫ��ĩ����Ϊľ̿�ۡ������е�һ�ֻ����ֵĻ���������������ľ̿�����ʵIJ�𣬶Թ����ĩ����ɽ��м��飻
��1�����������ڳ�ʪ�Ŀ����п���������ˮ������Ӧ���γɺ���ɫ�����⣬���ʧЧ����к���ɫ��
��2���������ڸ��������¿���CO��Ӧ���������Ͷ�����̼����ˣ��ɰ�ʧЧ�ķ�ĩ�ڸ�������CO��Ӧ������������
����𡿽⣺����BΪ��ɫ���壬DΪ��ɫ���嵥�ʣ���B����Ϊ����ͭ��D����Ϊͭ��A���л�ԭ�ԣ�������ͭ��Ӧ������C�ܹ���A��Ӧ����E�����A��̼�����ɵ�CΪ������̼��������̼����̼��Ӧ����һ����̼��EΪһ����̼��FΪ��ɫ���壬����һ����̼��Ӧ����FΪ�����������ɵ�GΪ����������ͭ�η�Ӧ����ͭ��H��������ͭ����ˣ�
��1�����ݷ�����֪��B������ͭ��F�������������CuO��Fe2O3��
��2����Ӧ����̼������ͭ�ڸ��µ������·�Ӧ����ͭ�Ͷ�����̼���仯ѧ����ʽΪC+2CuO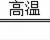
 2Cu+CO2�����÷�Ӧ�ǵ��ʺͻ��������ɵ��ʺͻ�����ķ�Ӧ�������û���Ӧ����Ӧ���Ƕ�����̼��̼��Ӧ����һ����̼�Ĺ��̣��仯ѧ����ʽΪCO2+C
2Cu+CO2�����÷�Ӧ�ǵ��ʺͻ��������ɵ��ʺͻ�����ķ�Ӧ�������û���Ӧ����Ӧ���Ƕ�����̼��̼��Ӧ����һ����̼�Ĺ��̣��仯ѧ����ʽΪCO2+C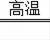
 2CO��
2CO��
��3����Ӧ����̼������ͭ�ڸ��µ������·�Ӧ����ͭ�Ͷ�����̼���÷�Ӧ�ǵ��ʺͻ��������ɵ��ʺͻ�����ķ�Ӧ�������û���Ӧ����Ӧ����һ����̼���������ķ�Ӧ�����û���Ӧ���ʷ�Ӧ���Ͳ�ͬ������û���Ӧ����ͬ��
����ľ̿����������ͭ������Ӧ�����ۿ����û�������ͭ�е�ͭ����ˣ���������������ͭ��Һ���ַ�ĩ��ɺ�ɫ����ʱ������˵������1��������
����ľ̿������ϡ���ᷴӦ�����ۿ���ϡ���ᷴӦ�γ�����������Һ��ͬʱ�ų���������ˣ��Ѻ�ɫ��ĩ��������ϡ����ʱ�����ֺ�ɫ��ĩ�ܽⲢ�ų�����ʱ����˵������2���������������������۶���������ľ̿�ۣ��ô�������ʱ���ַ�ĩȫ�������𣬿�˵������3��������
�ʴ�Ϊ��
ʵ����� ʵ������ �����Ƿ����
ʵ��1 ��ɫ��ĩ��ɺ�ɫ
ʵ��2 ��ɫ��ĩ���ܽ⣬�д��������ݲ���
ʵ��3 ����3��������
��1�����ڳ�ʪ�Ŀ�����������γɺ�ɫ�������ʧЧ���������Ҫ�ɷ�Ϊ���������仯ѧʽΪFe2O3��
�ʴ�Ϊ����������е�������ˮ��Ӧ���������⣻Fe2O3��
��2��ʹʧЧ�ķ�ĩ�����������ڸ��������£�ʹ��CO��ԭ���������ȵ����ۣ��ﵽ����������Ŀ�ģ����仯ѧ����ʽΪ��3CO+Fe2O3
 2Fe+3CO2��
2Fe+3CO2��
�ʴ�Ϊ��
��1��CuO�� Fe2O3����2��C+2CuO
 2Cu+CO2���� CO2+C
2Cu+CO2���� CO2+C
 2CO����3���û���Ӧ����
2CO����3���û���Ӧ����
������� �������� �����Ƿ����
ʵ��1 ��ɫ��ĩ��ɺ�ɫ
ʵ��2 ��ɫ��ĩ���ܽ⣬�д��������ݲ���
ʵ��3 ������
�������뷴˼����1����������е�������ˮ��Ӧ���������⣬Fe2O3����2��3CO+Fe2O3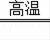
 2Fe+3CO2
2Fe+3CO2
������������Ϊ��ͼʽ�ƶ��⣬�ڽ������ʱ�����Ƚ������������������Ƴ���Ȼ�����Ƴ������ʺ����е�ת����ϵ�Ƶ�ʣ������ʣ�����Ƴ��ĸ������ʴ���ת����ϵ�н�����֤���ɣ�
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С��ͬѧѧϰ���������ƺ��������ƵĻ�ѧ���ʺ�֪�����������ƺ��������ƶ����������̼��Ӧ��������̼ͨ�����ʯ��ˮ���ܲ������Ե���������С��ͬѧ�����һ������
С��ͬѧ���뵽�ڡ�������ܽ��ھƾ��С���ʵ��ʱ���ƾ���Ϊ�ܼ����ܽ����⡣�ƾ��ܷ��ܽ��������ƺ�̼�����أ�С���������ϲ�ͨ��ʵ���֪���������Ʋ���ƾ�������ѧ��Ӧ�������ܽ��ھƾ����γ���ɫ������Һ���������Ƶľƾ���Һ���������Ƶ�ˮ��Һ��ѧ�������ƣ�̼���Ʋ���ƾ���ӦҲ�����ھƾ������ˣ�С��ͬѧ���Լ�������������������Ľ������
��1��С��ͬѧ�Ľ�������� 
��2��д������������漰�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�й����������У����ڻ�ѧ���ʵ��ǣ�������
A���е��絼���� B����������չ��
C����������ɫ���� D���ڳ�ʪ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������þ��п�������ֽ����ֱ�����ʵ�����������ͬ������ϡ���ᷴӦ���ܹ���ȷ��ӳʱ�䣨t���Ͳ�������������m����ϵ�������ǣ�������
A��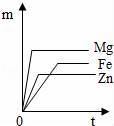
 B��
B��

C��
 D��
D��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
оƬ�����е��ԡ������ܼҵ硱�ĺ��IJ����������Ըߴ��ȵĵ��ʹ裨���Ԫ�ط���ΪSi��Ϊ�����Ƴɵģ��û�ѧ�����Ƶøߴ���ķ�Ӧԭ��Ϊ����SiO2+2C��Si+2CO�� ��Si+2Cl2��SiCl4 ��SiCl4+2H2��Si+4HCl��
��ش�
��1��������Ӧ�������û���Ӧ��������������ţ���
��2����Ӧ����̼���ʱ����������ԣ����������ԭ������
��3����Ӧ���������о綾��CO������������ѪҺ�е�Ѫ�쵰��ϣ�ʹѪ�쵰�ײ��ܺܺõ���������ϣ��Ӷ�ʹ����ȱ������ɡ�ú���ж���������������ˡ�ú���ж������㽫������Щ���δ�ʩ����ֻ�ش�һ�ּ��ɣ�����SiO2+2C�TSi+2CO����SiC14+2H2�TSi+4HC1������Ӧ�з�Ӧ��������ﶼ��һ�ֵ��ʺ�һ�ֻ���������û���Ӧ��
��2����Ӧ����̼���ʵõ���������������Ӧ���ǻ�ԭ�������л�ԭ�ԣ�
��3�����δ�ʩ�У�����ͨ����һ����̼����ɢʧ��120���ȵ绰��ȣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ɽ��ˮ�㣬���й��ĺ�����������У����Ͻ�Լ�����ܡ����š����۵��ǣ�������
A������ϯ B���ʻ�ɨĹ C����ҵ���� D��ȼ�ű���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʯ��ʯī��C60������̼Ԫ����ɵĵ��ʣ����й���̼�ĵ��ʵ�������ȷ���ǣ�������
A�����Ǻ�ɫ����
B���������г��ȼ��ʱ�����ɶ�����̼
C��̼ԭ�ӵ����з�ʽ��ͬ
D��һ�������£�ʯīת���ɽ��ʯ�������仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ˮ��ʵ��װ����ͼ��ʾ������ͼ�ش�
��1����ԴA��������
��2�����ռ���c����11.2mL����������Ӧ�ռ���d�����Լ����mL
��3���÷�Ӧ�Ļ�ѧ����ʽ��������
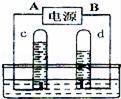

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NaNO3��KNO3���ܽ�����ݼ��ܽ���������£��� ��˵����ȷ����
��˵����ȷ����
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | |
| �ܽ��S/g | NaNO3 | 73 | 87.6 | 103 | 122 |
|
| KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 |
A��30��ʱ��������Һ����������������NaNO3>KNO3
B���ұ�ʾKNO3�ܽ������
C��t1��ʱNaNO3��KNO3���ܽ����ȣ���ʱ�¶�Ϊ
60��80��֮��
D����Ӱ��NaNO3��KNO3����Һ��Ϊ������Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com