
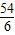 =
=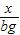
 2Fe+Al2O3
2Fe+Al2O3 


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ȷ��ij���ȼ���������������������ɣ��ֱ��������ʵ�顣��֪�����������ڸ����·�Ӧ���������������������������Ƶ�ˮ��Һ��Ӧ����ƫ�����ƣ�NaAlO2����������
��1����ȡag��Ʒ�������м�������������������Һ��������ɵ�����Ϊbg����Ӧ�Ļ�ѧ����ʽΪ ����Ʒ����������Ϊ g��
��2����ȡag��Ʒ��ʹ���ڸ�����ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ
����Ʒ��������������������Ϊ ��
��3������2���з�Ӧ������ȴ�������м������������ᣬ������ɵ���������Ϊcg����������루1�������õ������������c��b�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007�꽭��ʡ��ͨ�к�����ʵ��ѧУ���꼶��ѧ����ѡ�����Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com