��2006?������һģ�������dz�����ʵ��װ��ͼ
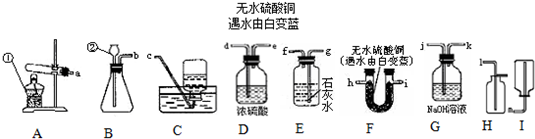
��1��ͼ���б�ŵ����������ǣ���
�ƾ���
�ƾ���
��
����©��
����©��
��2����ȡ���ռ������CO
2���壬ѡ�õ�װ�������
BDH��BFH
BDH��BFH
����װ����ĸ���ţ���ͬ���������Ƿ��ռ����ķ�����
��ȼ�ŵ�ľ������ƿ�ڣ��۲��Ƿ�Ϩ��
��ȼ�ŵ�ľ������ƿ�ڣ��۲��Ƿ�Ϩ��
����ȡ������Ļ�ѧ����ʽ��
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
��С�����齫ϡ���ỻ��Ũ���ᣬ����Ϊ�˷���������
������
������
�����������������������
Ũ������лӷ��ԣ�ʹCO2�л���HCl����
Ũ������лӷ��ԣ�ʹCO2�л���HCl����
��
��3��ij��ѧ�о���С���ͬѧѡ������װ�öԳ������ʽ�����̽����
̼����泥�NH
4HCO
3����һ�ֳ�����
��
��
�ʣ������ֽ��������ֻ��������һ���ǰ�����NH
3���������ֿ�����
CO2
CO2
��
H2O
H2O
��Ϊ��֤���˲��룬ѡ����������װ�ã����ӿڵ�����˳����a��
h
h
��
i
i
��
g
g
��
��4������H
2O
2��MnO
2��������Ӧѡ��
B
B
��Ϊ����װ�ã��仯ѧ��Ӧ����ʽΪ
��
�Դ�ʵ�飬�����Ǽ���˼�����о��������⣺
����٣�����MnO
2�������Է�Ӧ������û��Ӱ�죬�ҵ�ʵ�鷽���ǣ�ÿ�ξ���30mL10%��H
2O
2��Һ�����ò�ͬ��MnO
2��ĩ���������ⶨ�����ռ���500mL����ʱ���õ�ʱ�䣬������£�������ʵ����������ͬ��
| ʵ����� |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
| MnO2��ĩ������g�� |
0.1 |
0.2 |
0.3 |
0.4 |
0.5 |
0.6 |
0.7 |
0.8 |
| ����ʱ�䣨t�� |
17 |
8 |
7 |
5 |
4 |
3 |
2 |
2 |
��������������ݻش�MnO
2�������Է�Ӧ��������Ӱ�죬����У�����Ӱ�죿
��
��Ӱ�죬��һ����Χ�ڣ�MnO2������Խ��ӦԽ��
��Ӱ�죬��һ����Χ�ڣ�MnO2������Խ��ӦԽ��
��
����ڣ�H
2O
2��Һ���������������Է�Ӧ������û��Ӱ�죬���ʵ�鷽���ǣ�
��
ÿ���õ�����MnO2������������ͬ��������������H2O2��Һ����ʵ�飬�۲��ռ���������ʱ�����ĵ�ʱ��
ÿ���õ�����MnO2������������ͬ��������������H2O2��Һ����ʵ�飬�۲��ռ���������ʱ�����ĵ�ʱ��
��
����ۣ�������Щ���ؿ���Ӱ��÷�Ӧ�������أ�
��˵�����һ�����룺
MnO2������С�й�
MnO2������С�й�
��
����ܣ���֪�����ڸ�ʵ�������µ��ܶ�Ϊ1.28g/L����Ҫ��ȡ250mL����������������������Ҫ����30%��H
2O
2��Һ���ٿˣ������ԭ��������H-1O-16��




