

 K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2���� Cu+H2O��
Cu+H2O��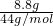 =0.2mol
=0.2mol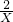 =
=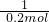
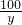 =
=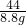 y=20g
y=20g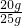 ��100%=80%
��100%=80% K2MnO4+MnO2+O2����H2+CuO
K2MnO4+MnO2+O2����H2+CuO Cu+H2O��
Cu+H2O��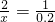



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| �Ƚ���Ŀ | ��ˮ�� | �����ſ����� |
| �ռ����������Է��� | CO2�������ɺʹ�ˮ���ݳ����ٶ�Զ�������ܽ����ˮ��Ӧ���ٶ� | CO2�ܶȱȿ��� �� �� ���Ҳ���������Ӧ |
| �ռ����̷��� | �������� ����ƿ����ð���� ����ƿ����ð���� |
������������������ɫ���ʼ�������ȷ����������ȼ��ľ���ƽ������ڻ���Ϩ��Ҳ��֤������ȫ�ž� |
| ���ռ���CO2�ļ���ƿ�е���������������ʯ��ˮ���� | �Ȼ��Ǻ��������ʱ��϶� | �Ȼ��Ǻ��������ʱ��ϳ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com