��2013?�껨̨��һģ����������������?Ŭ�����Ƿ�����һ���кʹ���������̼���·������ǽ�������̼�ӻ������糧�������з�����������뺬�г�ʯ�ɷֵ�ˮ��Һ������кʹ������ڷ�Ӧ�����У���ʯ�ijɷֲ��������ն�����̼��ͬʱ��������һЩ���õĸ���Ʒ����Щ����Ʒ��������������ԭ���ϺͲ�������ҵ������Ҫ���̿�ʾ����ͼ��
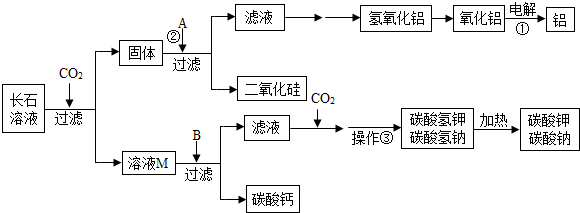
��1�������ҽ�CO
2�ӻ������糧�������з��������ͨ�뺬�г�ʯ��ˮ��Һ�����գ��ӻ����ĽǶȿ�����������������
���ٶ�����̼�ŷţ���������ЧӦ
���ٶ�����̼�ŷţ���������ЧӦ
��
��2����ʯ�ǵؿ�������Ŀ�ʯ�������ߴ�60%����ʯ��Ҫ�����س�ʯ��KAlSi
3O
8�����Ƴ�ʯ��NaAlSi
3O
8�����Ƴ�ʯ��CaAl
2Si
2O
8����l t���Ƴ�ʯ�γɵ�ˮ��Һ������320kg CO
2�����Ƴ�ʯ����CO
2��Ӧ����ʽΪ
CaAl
2Si
2O
8+2CO
2+4H
2O=Ca��HCO
3��
2+2SiO
2��+2A1��OH��
3������ģ�¸Ƴ�ʯ��д���Ƴ�ʯ����CO
2�Ļ�ѧ��Ӧ����ʽ
NaAlSi3O8+CO2+2H2O�TNaHCO3+3SiO2��+Al��OH��3��
NaAlSi3O8+CO2+2H2O�TNaHCO3+3SiO2��+Al��OH��3��
��
��3��д����Ӧ�ٵĻ�ѧ����ʽ
����A��pHֵС��7����д��һ�����Ϸ�Ӧ�ڵĻ�ѧ����ʽ
Al��OH��3+3HCl�TAlCl3+3H2O
Al��OH��3+3HCl�TAlCl3+3H2O
��
��4����ҺM�е�����Ϊ
KHCO3��NaHCO3��Ca��HCO3��2
KHCO3��NaHCO3��Ca��HCO3��2
��B����������������������BΪ
��ʯ�ң���Ca��OH��2����
��ʯ�ң���Ca��OH��2����
����������ҪĿ��Ϊ
����Һ����ȡ���壨�𰸺������ɣ�
����Һ����ȡ���壨�𰸺������ɣ�
��




 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�