
| ��� | ����ϡ���������/g | ʣ���������/g |
| ��һ�� | 10 | 5.5 |
| �ڶ��� | 10 | m |
| ������ | 10 | 1.2 |
| ���Ĵ� | 10 | 1.2 |
���� ��1���Ƚϵ�һ�κ͵����ε����ݿ�֪��һ����������ȫ��Ӧ������̼���8g-5.5g=2.5g����˵ڶ�����Ҳ������2.5g̼��ƣ������õ�һ�ε�ʣ�����������ȥ�ڶ������ĵ�̼��Ƶ��������ǵڶ���ʣ������������
��2���Ƚϵ����κ͵��Ĵε����ݿ�֪����Ʒ�����ʵ�����Ϊ1.2g�������Ʒ�������Ϳ��Լ������Ʒ��̼��Ƶ�����������
��3�������������Ƶ�������ʯ��ʯ��̼��Ƶ���������������̼��Ʒֽ�Ļ�ѧ����ʽ�Ϳ��Լ������Ҫʯ��ʯ��������
��� �⣺
��1���ϵ�һ�κ͵����ε����ݿ�֪��һ����������ȫ��Ӧ������̼���8g-5.5g=2.5g����˵ڶ�����Ҳ������2.5g̼��ƣ������õ�һ�ε�ʣ�����������ȥ�ڶ������ĵ�̼��Ƶ��������ǵڶ���ʣ������������m=5.5g-��8g-5.5g��=3g
��2���Ƚϵ����κ͵��Ĵε����ݿ�֪����Ʒ�����ʵ�����Ϊ1.2g����Ʒ��̼��Ƶ���������Ϊ$\frac{8g-1.2g}{8g}$��100%=85%
��3������Ҫʯ��ʯ������Ϊx��
CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2��
100 56
x��80% 280Kg
$\frac{100}{x��80%}=\frac{56}{280Kg}$
x=625Kg
�𰸣�
��1��3��
��2��85%��
��3����Ҫʯ��ʯ������Ϊ625kg��
���� ������Ҫ���麬�������ʵĻ�ѧ����ʽ�ļ��㣬�ѶȽϴ�����ʱҪע�⣺��ѧ����ʽҪд��ȷ��ʼ�ղ�Ҫ���������غ㶨�ɣ���������Ҫ�����ڼ������У�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
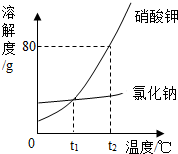 ��ͼ���Ȼ���������ص��ܽ�����ߣ��ش�
��ͼ���Ȼ���������ص��ܽ�����ߣ��ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ͻ��������ֻ��������Ͻ����ۺ϶��ɵľ��н������Ե����� | |
| B�� | ���ճ������У�����ʹ�õij������Ǵ��������������ǵĺϽ� | |
| C�� | ͭ�Ļ�ѧ���ʲ����ã�����ͭ��Ʒ�������� | |
| D�� | ������»�����˵���ƽ��Ӳ�ȷdz��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
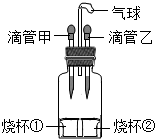 ��ͼ��ʾ�����ιܼ��е�Һ�強�����������Թ���һ��ʱ���ָ�ԭ״���ٽ��ι����е�Һ�強�������������Թ����Ҳ��ָ�ԭ״����ιܼס��Һ��ձ��٢��е����ʿ����ǣ�������
��ͼ��ʾ�����ιܼ��е�Һ�強�����������Թ���һ��ʱ���ָ�ԭ״���ٽ��ι����е�Һ�強�������������Թ����Ҳ��ָ�ԭ״����ιܼס��Һ��ձ��٢��е����ʿ����ǣ�������| A�� | �ף�ˮ���٣�ʳ�Σ� �ң�ˮ���ڣ����� | |
| B�� | �ף�ˮ������泥� �ң�ϡ�ν����ڣ��� | |
| C�� | �ף�ˮ���������ƣ� �ң�ϡ���� �ڣ�п | |
| D�� | �ף�ˮ����ʯ�ң� �ң�ϡ���� �ڣ��������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com