
��ͼ��A~G�dz��л�ѧ�г����İ������ʣ���������ʾ���������ܷ�����Ӧ����������ʾ���ʼ����ת����ϵ����Ӧ��������ȥ������A�Ǻ�ɫ���壬C����ɫ��ζ���ж����壬C��D��E�Ƿǽ����������ش��������⡣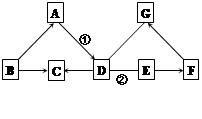
��1��G�Ǻ�����Ԫ�صļд������G��һ����; ��
��2��D�Ļ�ѧʽΪ ��
��3����Ӧ�ٲ����ڻ�����Ӧ���ͣ�������ұ
��ҵ����д��һ����������Ļ�ѧ����ʽ ���� �� ��
��4����ҵ�����г���F�������᳧��ˮ�е����ᣬ��Ӧ�ڵĻ�ѧ����ʽΪ ��
��1��������������𰸺������ɣ�����2��CO2 ����3��3CO+ Fe3O4����3Fe + 4CO2����CO+ CuO ��Cu + CO2���� ��4��CO2+ H2O = H2CO3
�������������A�Ǻ�ɫ���壺����Ϊ���ۻ�ľ̿��������ͭ����������������C����ɫ��ζ���ж����壬����Ϊһ����̼��1��G�Ǻ�����Ԫ�صļΪ�������ƣ�G����D��C���� D����Ϊ������̼����3����Ӧ�ٲ����ڻ�����Ӧ���ͣ�������ұ��ҵ����AΪ��ɫ���壬DΪ������̼����Ϊһ����̼������ͭ��������������������ԭ��Ӧ����ѡһ�����ɣ���4����ҵ�����г���F�������᳧��ˮ�е����ᣬ��FΪ�������ƣ�E�Ƿǽ��������EΪˮ���������Ʒ�Ӧ����F�������ƣ��ʢ�Ϊ������̼��ˮ�ķ�Ӧ��
���㣺���ʵ��ƶϣ���ѧ����ʽ����д��̼���仯���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
��������SO2����ͨ���������һ����ɫ���д̼�����ζ���ж����壬����������������Һ��Ӧ��������ˮ������ij̽��ʵ��С����������ͼװ�ú�ҩƷ��ȡ��������̽������������ˮ��ͨ��������ܷ�����ѧ��Ӧ����Ƶ�̽���������£�����ش����е��й����⣺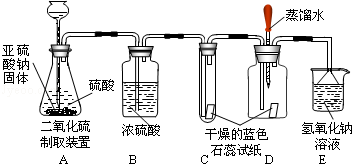
��1�����裺SO2��ˮ��ͨ��������ܷ�����ѧ��Ӧ����������һ���ᣮ
��2����Ʒ���������֤ˮ�ܷ�ʹ��ɫʯ����ֽ��ɫ������֤SO2�����ܷ�ʹ�������ɫʯ����ֽ��ɫ�������֤SO2�����ܷ�ʹʪ�����ɫʯ����ֽ��죮
��3���������ϣ�����Ϊ���о�С����Ҫ���ĵ�����������Ӧ���������е� ����д��ţ���
��SO2������ˮ��������ʹʪ�����ɫʯ����ֽ��죬��SO2�ܱ�Ũ������
��4��ʵ�飺
��ʵ������У�װ��C��ʯ����ֽ����ɫʼ��û�б仯����˵�� ��
��װ��D�н�ͷ�ι��е�����ˮ��SO2��������֮ǰ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯������SO2����ͨ��ʱ������ʪ�����ɫʯ����ֽ��죬������˵�� ��
��װ��E�������� ��д��������Ӧ�Ļ�ѧ����ʽ ��
��5�����ۣ�ԭ���� �����������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
(4��)A��B��C��D��EΪ�������ֳ������ʣ� ����A�����������Һ�壬 B��D�����壬ֻ��E������Ԫ�أ�B�dz������ʣ���Ӧ�������������ʾ�����ȥ������������ʾ������һ��ת��������������ʾ�������ʼ���Է�����Ӧ,���з�Ӧ���dz��г�����Ӧ��
��д�����ʻ�ѧʽ��A�� ��C�� ��
��д����ѧ����ʽ��D+E ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
��6�֣���ͼ��ʾΪ������֮���ת����ϵ����ش��������⣺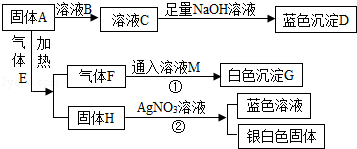
��1��д��������ĸ���������ʵĻ�ѧʽ��A���� �� D���� �� E���� ����
��2��д�����̢ڵĻ�ѧ����ʽ�� �����������Ӧ�������� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
��֪��ĩX���������������е�һ�֣�ȡ���ȷݸ÷�ĩ���ֱ���뵽������̼������Һ����������Һ��ϡ�����в����������������±�����ù����ĩX��
| �������� | ̼������Һ | ��������Һ | ϡ���� |
| �� �� | ������ɫ���� | ������ɫ���� | �����ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
��6�֣�ijʳ����Ʒ�к�������ɳ�����Ȼ��ƺ��Ȼ�þ�������dz�ȥʳ����Ʒ��ɳ�����Ȼ��ƺ��Ȼ�þ��ʵ�����̣�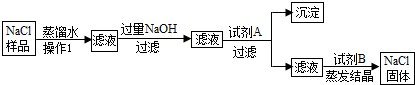
��������ͼ�ش�
��1��������������� ��������Լ�A�� ��������
��
��2��д��NaOH�����ʷ�Ӧ�Ļ�ѧ����ʽ ��
��3�������Լ�B��Ŀ���� ��
��4�������ᾧʱ�õ��������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
�������ñ��ǣ���Ҫ�ɷ���̼��ƣ����ʲ��μӷ�Ӧ�Ҳ�����ˮ���ʹ���Ϊԭ
����ȡ�ռijС��ͬѧ��ͼ��ʾ���̽���ʵ�飬����������۲��ش�������⡣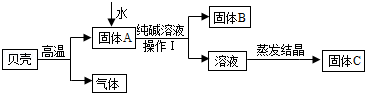
��1�����Ǹ�������ʱ��������Ӧ�Ļ�ѧ����ʽ��________________________________��
��2��ˮ���뵽����A�еķ�Ӧ��________������ȡ����ȡ��������������õ��IJ���
�������ձ���________��������������Һ�����ᾧ��________________ʱֹͣ���ȡ�
��3��������C�����Һ������Һ��һ�����е�������________��д�������ʵ����е�һ����;________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
(7��)������һЩ�������ʼ以��ת���Ĺ�ϵͼ(���ַ�Ӧ������ʡ��)����֪A��B��X��Y��Ϊ��ɫ���壬E��FΪ�������ʡ�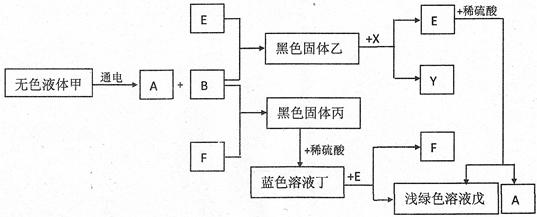
��ش�
��1��д���������ʵĻ�ѧʽ��F ���� ��
��2��д������ת���Ļ�ѧ����ʽ��
�� �ס�A��B ��
�� �ң�X��E��Y ��
��3��˵����ɫ��Һ�������ʵ�һ����; ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
��ͼ��ѧ��Ӧ����Һ��ɫ�仯�����ˡ�ħ�����磬������ѧ������ش�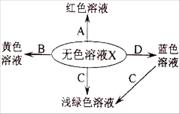
��1����ҺX�� ����ᡱ����������Ρ�����
��2����X��ϡ���ᣬB���������X��B��Ӧ�Ļ�ѧ����ʽΪ ��
��3����X��ϡ���ᣬC�ǵ��ʣ���C�������� ��
��4����X��ϡ���ᣬD�Ǽ��X��D��Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com