25��ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ����������ż���㷺��Ӧ�ã�
��1������ˮ���������̴�������ͼ��
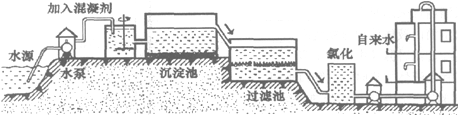
��ͼ���˳����л���̿�㣬����̿��
����
���ã��ù����з���
����
�仯���Ȼ�ʱ��ͨ��ͨ��һ��������������ˮ��Ӧ��������ʹ����ᣮʵ��������AgN03��Һʱ����ʹ������ˮ����ԭ���ǣ��û�ѧ����ʽ��ʾ��
AgN03+HCl=AgCl+HNO3
��������أ�K
2Fe0
4����һ�������ˮ�������������������Ԫ�صĻ��ϼ���
+6
��
��2�����ˮʱ����������NaOH������ǿˮ�ĵ����ԣ��ֽ�0.1gNaOH�ܽ���99.9gˮ���ֱͨ����Դ�����Դ����������һ�˷ų���������
O2
������Һ��NaOH������������Ϊ0.2%ʱ����
50.0g
ˮ���ֽ⣮
��3���ں����мס��ҡ�����������������λ������ͼ��ʾ����ÿ�������ų��ķ�Һֻ����Na
2C0
3��FeCl
3��NaOH��
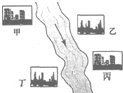
HCl�е�һ�֣�ij����С��Ժ�ˮ���ʱ���֣��״���ˮ����ɫ���Ҵ���ˮ�ʺ��ɫ��������ˮ�ɻ���壻�����������ݣ���ˮ���壮��ش�
�ټ����ų��ķ�Һ��һ�ּ������ʣ���������ʳʼ��Կ�ѡ�õ��Լ���
��̪��ʯ��
��
���Ҵ���ˮ�ʺ��ɫ�Ļ�ѧ����ʽ��
3NaOH+FeCl3=Fe��OH��3��+3NaCl
��
�۶����������ݵĻ�ѧ����ʽ��
Na2C03+2HCl=2NaCl+C02��+H20
��
��4������ˮ��Դ������Ӧ�ò�ȡ�Ĵ�ʩ��
��ҵ�����м�����������ˮ����Ⱦ��IJ�������ũҵ������ʹ�ø�Ч�Ͷ���ũҩ�������������ƹ�ʹ������ϴ�·۶���ˮ���д����������ŷţ���ֻҪ���һ�㼴�ɣ�
��



 ��У����ϵ�д�
��У����ϵ�д�

 HCl�е�һ�֣�ij����С��Ժ�ˮ���ʱ���֣��״���ˮ����ɫ���Ҵ���ˮ�ʺ��ɫ��������ˮ�ɻ���壻�����������ݣ���ˮ���壮��ش�
HCl�е�һ�֣�ij����С��Ժ�ˮ���ʱ���֣��״���ˮ����ɫ���Ҵ���ˮ�ʺ��ɫ��������ˮ�ɻ���壻�����������ݣ���ˮ���壮��ش�