
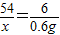 ЃЛx=5.4gЃЌдђбѕЛЏТСЕФжЪСПЪЧ15.6g-5.4g=10.2g
ЃЛx=5.4gЃЌдђбѕЛЏТСЕФжЪСПЪЧ15.6g-5.4g=10.2g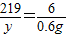 ЃЌy=21.9g
ЃЌy=21.9g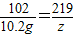 ЃЌдђz=21.9gЃЌгЩгыЗДгІКѓШмвКжажЛКЌгавЛжжШмжЪЃЌдђ50gбЮЫсвВЪЧЧЁКУЗДгІЃЌдђ
ЃЌдђz=21.9gЃЌгЩгыЗДгІКѓШмвКжажЛКЌгавЛжжШмжЪЃЌдђ50gбЮЫсвВЪЧЧЁКУЗДгІЃЌдђ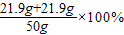 =87.6%
=87.6%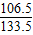 =ЃЈ21.9g+21.9gЃЉ×
=ЃЈ21.9g+21.9gЃЉ×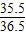 ЃЌw=53.4gЃЌЫљвдЯђЗДгІКѓШмвКжаМгШы35gЫЎЃЌМгЫЎКѓШмвКжаШмжЪЕФжЪСПЗжЪ§ЮЊ
ЃЌw=53.4gЃЌЫљвдЯђЗДгІКѓШмвКжаМгШы35gЫЎЃЌМгЫЎКѓШмвКжаШмжЪЕФжЪСПЗжЪ§ЮЊ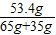 ×100%=53.4%ЃЛ
×100%=53.4%ЃЛ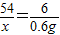 ЃЛЃЈ3ЃЉ87.6%ЃЛЃЈ4ЃЉ53.4%ЃЛЃЈ5ЃЉ25ЃЛ
ЃЛЃЈ3ЃЉ87.6%ЃЛЃЈ4ЃЉ53.4%ЃЛЃЈ5ЃЉ25ЃЛ


| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
| 54 |
| x |
| 6 |
| 0.6g |
| 54 |
| x |
| 6 |
| 0.6g |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃКНтД№Ьт
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com