


 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ�ʵ����
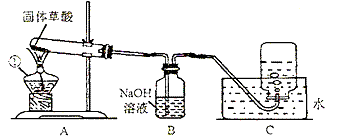
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
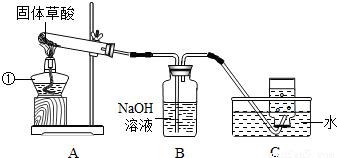
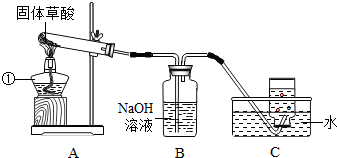
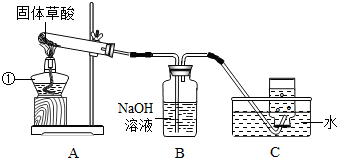
ij����С�����ò���ֽ���ȡCO���塣��֪�������ȷֽ�Ļ�ѧ����ʽΪH2C2O4?2H2O=CO��+CO2��+3H2O����С���ͬѧ����������ͼ��ʾ��ʵ�顣��ش�:
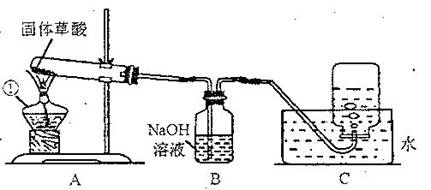
(1)ͼ�������ٵ��������������� ����װ��װ�ú�Ӧ�Ƚ��еIJ����������������� ��
��2��Bװ�õ��������������������� ��
��3��CO�ռ���������װ��ʱӦ�������������� ��
��4��Ϊ����֤����ֽ�IJ��ʹ��������ҩƷ���ٳ����ʯ��ˮ�������ȵ�����ͭ������ˮ����ͭ���壨ע����������ˮ�ɰ�ɫ��Ϊ��ɫ����������������Һ�������ֽ����ͨ���Լ�����ȷ˳��Ӧ�������������� ������ĸ����
A. �٢ڢۢܡ� B. �ۢܢڢ١� C. �ۢ٢ڢ١� D. �ܢۢڢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com