�⣺��1���÷�Ӧ�����ɶ�����̼���壬Ϊ���������̼����Ĵ��ڣ�����B�е��Լ��dz���ʯ��ˮ���ʴ�Ϊ������ʯ��ˮ��
��2���ڸ��������£�̿���������̼��Ӧ������һ����̼������һ����CO�����ֿɼ��л��棬���еĻ������ʹ�¶ȸ��ߣ��ʴ�Ϊ��CO���γɸ���������
��3����Ӧ����̿������ͭ����Ӧ�����Ǹ���д����ѧ��Ӧʽ��C+2CuO

2Cu+CO
2����
�ʴ�Ϊ��C+2CuO

2Cu+CO
2����
��4�����ɵĶ�����̼������42-37.6=4.4g
�裺����4.4g������̼��Ҫ����ͭXg
C+2CuO

2Cu+CO
2��
160 44
X 4.4
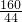
=

X=16g
����16������ͭ�μ��˷�Ӧ��
��������1�����ݸ÷�Ӧ�����ɶ�����̼���忼�ǣ�B���Լ������ƣ�
��2��̿���������̼��Ӧ������һ����̼�������ֿɼ��л��濼�ǣ�
��3�����ݷ�Ӧ��ͷ�Ӧ����д����ѧ��Ӧʽ��
��4������̿��ԭ����ͭ�Ļ�ѧ��Ӧʽ�ͱ����е�������ݼ��㼴�ɣ�
���������⿼�黯ѧ��Ӧʽ����д����ؼ��㣬�����ڻ���֪ʶ����ͬѧ��Ҫ��̿�Ļ�ѧ�����н�Ϊϵͳ���˽�ſ����ô��⣮

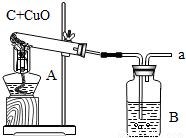
 ��ͼ�Ǹ�������ľ̿��ԭ����ͭ��ʵ��װ��ͼ
��ͼ�Ǹ�������ľ̿��ԭ����ͭ��ʵ��װ��ͼ 2Cu+CO2����
2Cu+CO2���� 2Cu+CO2����
2Cu+CO2���� 2Cu+CO2��
2Cu+CO2��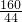 =
=



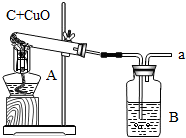 ��ͼ�Ǹ�������ľ̿��ԭ����ͭ��ʵ��װ��ͼ
��ͼ�Ǹ�������ľ̿��ԭ����ͭ��ʵ��װ��ͼ ��2007?�ɽ���һģ����ͼΪһ����������ľ̿�ۣ��Թ�������ԭ����ͭ��ʵ��װ��ͼ��
��2007?�ɽ���һģ����ͼΪһ����������ľ̿�ۣ��Թ�������ԭ����ͭ��ʵ��װ��ͼ�� �ڸ���ʱ��̼����ijЩ�����ﷴӦ������
�ڸ���ʱ��̼����ijЩ�����ﷴӦ������