
�����ѧϰ��������ἴ�̲ġ�����ѧ����ʳס�С�������Դ�����Ͽ�ѧ��ҽ�������ȷ���Խ��Խ��������Լ��ļ�ֵ��
��1��һ������ҩ������������ͭ��ȼ��ʱ������ɫ���棬��ѧ����ʽ���£�
2Cu��NO3��2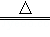 2CuO+O2+4X������X��һ�ֿ�����Ⱦ��仯ѧʽΪ ��
2CuO+O2+4X������X��һ�ֿ�����Ⱦ��仯ѧʽΪ ��
��2��������ԭ����һ����̼����������Ӧ��д���䷴Ӧ�Ļ�ѧ����ʽΪ ��
��3��С����̽�����ƽ����������Ƿ��н���ͭ������ѡ�������Լ��е� ��
A��ZnC12��Һ B��Mg��NO3��2��Һ C��H2SO4��Һ D��AgNO3��Һ
��4��Ϊ̽��������ȼ�յ���������ͬʱ��ȼСľ����Сú�飬����Сú���ȼ����ʱ�䳤��ԭ���� ��
��5���������������pHΪ6���ҵ������У���ij������pHΪ4�������������ֲ��ȣ����������п����������������Ե��� ��
A����ʯ�� B����ʯ�� C��������� D���������ƣ�
��1��NO2����2��3CO+Fe2O3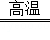 2Fe+3CO2����3��D����4��B��
2Fe+3CO2����3��D����4��B��
���������������1���ɷ�Ӧ�Ļ�ѧ����ʽ2Cu��NO3��2�T2CuO+O2��+4X�����ɵ�֪��Ӧǰ���ԭ�ӵĸ���Ϊ��
��Ӧǰ��Ӧ��
ͭԭ�� 2 2
��ԭ�� 4 0
��ԭ�� 12 4
���ݻ�ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬������X��4�������к���4��Nԭ�Ӻ�8��Oԭ�ӣ���ÿ��X������1��Nԭ�Ӻ�2��Oԭ�ӹ��ɣ�����X�Ļ�ѧʽΪNO2��
��2��һ����̼����������Ӧ���ɶ�����̼����������ʽΪ��3CO+Fe2O3 2Fe+3CO2��
2Fe+3CO2��
��3���������ǿ�Ľ����ܹ��ѽ���������Ľ�����������Һ���û�������������п��þ�Ļ�Ա�ͭǿ������ͭ�������Ȼ�п������þ��Ӧ����˲���ȡ��ͭ�Ļ�������������ͭҲ������ϡ���ᷴӦ�����Ҳ����ȡ��ͭ�Ľ�����Ա���ǿ������ͭ���û����������еĽ�������˿��������жϡ��ƽ����������Ƿ��н���ͭ��
��4�����pHԽ��������Խ����������Ҫ����ij������pHΪ4��Ϊ6�ķ����Ǽ����Լ��Ե����ʶ���������ģ�A����ʯ������ˮ�����ɼ��Ե��������ƣ����ù��̻�ų��������ȣ������������������ʧ�����Բ���ȡ��B�����������Լ��ԣ��ܹ��к����Ե�������������Ŀ��Ҫ��C��������Ƶijɷ����������ƺ�����ƻ��������ԣ��������������������ԣ�D�����������Լ��ԣ��ܹ������ᵫ�����̫ǿ����˲������ڸ���������������ѡB
���㣺�����غ㶨�ɼ���Ӧ�ã������Ļ�ѧ���ʣ��кͷ�Ӧ����Ӧ�ã���д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ��ȼ����ȼ�յ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��д�����з��ŵ�����
��K+ ��3OH- ��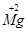
��2���û�ѧ�����ʾ��
��1����ԭ�� ��2������
��3��������̼���� �� ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ�ط��Ż�ѧʽ��ʾ
(1) 2����ԭ��___________��
(2) ����______________��
(3) 5��������__________��
(4) ���ֶ�����̼��ѧ���ʵ���С����________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����л�ѧʽ�����ֵĺ��壺
��1��3��������������___________��
��2��Mg2+��2�����___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͨ��һ��Ļ�ѧѧϰ������ѧ�������û�ѧ�����������������磺
��1���û�ѧ���ű�ʾ����2����ԭ�ӡ� ����2�������ӡ� ��
��2������������̣�MnO2�� ����Ԫ�صĻ��ϼ� ��
��3����ҵ���������������Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������ѧ���ӻ�ѧ����������¿γ̸ĸ�����������ͨ����һ�껯ѧ��ѧϰ�������û�ѧ֪ʶ���������е�����������г��ֵ����⣺
��1��Ϊ����ʹ���ǰ��Ե���˳��ڱ��棬����һ���ǽ���˷����չ��У����ϸǺ����ڸǵ���Χ��������ˮ��������Ŀ���� �Է���˱��ʣ�
��2����¯��������Ҫԭ���У�����ʯ����̿��ʯ��ʯ�ȣ���¯�ڷ�������Ҫ��ѧ��Ӧ�У�
�ٽ�̿ȼ��Ϊ�����ṩ���� ��
���Խ�̿Ϊԭ����ȡ��ԭ�� ��
�۸������û�ԭ����ԭ����ʯ���Դ�����Ϊ����������� ��
��3��ij�ؾ����鷢���������д���ʹ�ú���ú��Ϊȼ����������������ŷŴ����Ķ�����̼���������꣬������������ĸĽ���ʩ������ú������������ʯ�Ҽ����������ɵĶ���������ԭ�����û�ѧ����ʽ��ʾΪ ��
��4��Ҫϴȥ��ˮ�õ������ڱ��ϵ�ˮ��[��Ҫ��CaCO3��Mg(OH)2]���ɼ�������ȥ����صĻ�ѧ����ʽΪ��CaCO3+2CH3COOH=(CH3COO)2Ca+H2O+CO2����Mg(OH)2+2CH3COOH=(CH3COO)2Mg+2H2O��������Ĵ���ܹ�������Ϊ�ᷢ����Ӧ___ ______________________________��ʹ������ʴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ʵ��Ļ�ѧ���ź����ֻش�
��1��������ԭ��_________ ��
��2��þ����_________ ��
��3������ˮ�Ļ�ѧ���ʵ���С����_________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�û�ѧ�����ʾ��
��1��2����ԭ�� ��
��2��һ��þ���� ��
��3������������Ԫ�ػ��ϼ���+3�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���H��O��S��C��Mg����Ԫ����ѡ���ʺϵ�Ԫ�أ�������Ҫ���û�ѧ������գ�
��1��3������������� ��
��2��2��ԭ���� ��
��3��д������þ�н���Ԫ�صĻ��ϼ�����
��4���������� ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com