
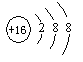 ����ͼ�Ľṹʾ��ͼ��ʾ���� ��д���ӷ��ţ���
����ͼ�Ľṹʾ��ͼ��ʾ���� ��д���ӷ��ţ���
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� ���� |
��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 2 | 3 Li � 6.941 |
4 Be ��? 9.012 |
5 B? ��? 10.81 |
6 C? ̼? 12.01 |
7 N? ��? 14.01 |
8 O? ��? 16.00 |
9 F? ��? 19.00 |
10Ne? ��? 20.18 |
| 3 | 11Na ��?22.99 |
12Mg þ?24.31 |
13 Al ��?26.98 |
14 Si ��?28.09 |
15 P? ��?30.97 |
16 S? ��?32.06 |
17 Cl? ��?35.45 |
18 Ar? �?39.95 |
 ����ʾ�����ӷ���Ϊ
����ʾ�����ӷ���Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 ����ʾ�����ӷ���Ϊ
����ʾ�����ӷ���Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ʾ�����ӷ���Ϊ
����ʾ�����ӷ���Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
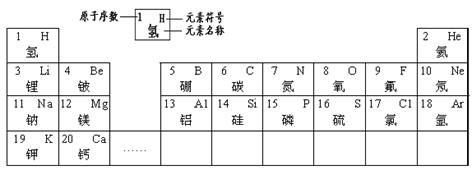
| 1 H �� |
2 He �� | ||||||
| 3 Li � |
4 Be �� |
5 B �� |
6 C ̼ |
7 N �� |
8 O �� |
9 F�� | 10 Ne �� |
| 11 Na �� |
12 Mg þ |
13 Al �� |
14 Si �� |
15 P �� |
16 S �� |
17 Cl �� |
18 Ar � |
 ����ʾ�����ӷ���Ϊ
����ʾ�����ӷ���Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com