ͭ�����������������й㷺ʹ�õĽ�����
��1����ҵ����һ����̼�ͳ�������Ҫ�ɷ�����������ұ��������Ӧ�Ļ�ѧ����ʽΪ
��
��2���������������
����������ˮ������ͬ���õĽ��
����������ˮ������ͬ���õĽ��
����������Գ�ȥ���⣨��Ҫ�ɷ����������������Ļ�ѧ����ʽ��
Fe2O3+6HCl=2FeCl3+3H2O
Fe2O3+6HCl=2FeCl3+3H2O
��
��3��ͭ����Ҳ����������ɫͭ�⣬ͭ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ����ѧʽΪCu
2��OH��
2CO
3�������Ԫ����
4
4
�֣�
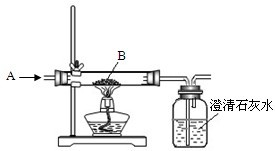
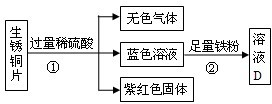
��4����ʦ�������ͭƬ�����ۺ�ϡ�������ʵ�飬ʵ�������ͼ5��ʾ����������������ȥ����

1����ɫ����C�Ļ�ѧʽΪ
Cu
Cu
��
2�����з����ķ�Ӧ�����û���Ӧ����д�����Ļ�ѧ����ʽ
Fe+CuSO4=Cu+FeSO4��Fe+H2SO4=FeSO4+H2��
Fe+CuSO4=Cu+FeSO4��Fe+H2SO4=FeSO4+H2��
����Ӧ��Һ�м��ٵ�������
ͭ���Ӻ�������
ͭ���Ӻ�������
�����ӵ�������
��������
��������
��


 2Fe+3CO2
2Fe+3CO2

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�