


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

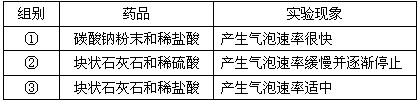
| ��� | ҩƷ | ʵ������ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| B���Σ� | C��� | D���ᣩ | |
| Һ������ | ̼��������Һ | ����ʯ��ˮ ����ʯ��ˮ | Ũ���� Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ��� | ҩƷ | ʵ������ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| | B���Σ� | C��� | D���ᣩ |
| Һ������ | ̼��������Һ | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
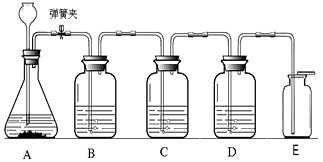
����װ������ʵ������CO2���Ʊ������������飬����ռ�һƿ�����CO2����ش��������⣺

��1����ʵ������ȡ������̼ҩƷѡ���̽��ʵ�飬��¼���£�
| ��� | ҩƷ | ʵ������ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
����ȡ���ռ��ĽǶȷ�����һ��ѡ��� ������ţ���ҩƷ����������Ӧ�Ļ�ѧ����ʽΪ ��
��2����C��D�е�Һ�����������±���
| B���Σ� | C��� | D���ᣩ | |
| Һ������ | ̼��������Һ |
��3��B�з�Ӧ�Ļ�ѧ����ʽ�� ��
��4��C�з�Ӧ�Ļ�ѧ����ʽ�� ��
��5����Ӧ�����н����ɼйرգ���A�п����������� ��
��6��E�ռ�����˵��������̼���е����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����װ������ʵ������CO2���Ʊ������������飬����ռ�һƿ�����CO2����ش��������⣺

��1����ʵ������ȡ������̼ҩƷѡ���̽��ʵ�飬��¼���£�
| ��� | ҩƷ | ʵ������ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
����ȡ���ռ��ĽǶȷ�����һ��ѡ��� ������ţ���ҩƷ����������Ӧ�Ļ�ѧ����ʽΪ ��
��2����C��D�е�Һ�����������±���
| B���Σ� | C��� | D���ᣩ | |
| Һ������ | ̼��������Һ |
��3��B�з�Ӧ�Ļ�ѧ����ʽ�� ��
��4��C�з�Ӧ�Ļ�ѧ����ʽ�� ��
��5����Ӧ�����н����ɼйرգ���A�п����������� ��
��6��E�ռ�����˵��������̼���е����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���п���ѧһģ�Ծ���1���������棩 ���ͣ������

| ��� | ҩƷ | ʵ������ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| B���Σ� | C��� | D���ᣩ | |
| Һ������ | ̼��������Һ | ______ | ______ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com