
����Ŀ��ijƷ��άC����Ƭ��ǩ��ͼ1��ʾ��Ϊ�о������ʲ��ⶨ����NaHCO3�������������̽�����̡�

��1��άC����Ƭ�������л�����г�ζ���ϡ������ᡢ_______��
��2����άC����ƬͶ��ˮ�У��۲쵽�д������ݲ�������Ҫ����Ϊ�����ᣨH3C6H5O7����NaHCO3������Ӧ�������Ƹ÷�Ӧ��
3NaHCO3+H3C6H5O7= Na3C6H5O7+3 H2O+_______��
��3��Ϊ�ⶨNaHCO3���������ͼ2װ�ã������������������

ʵ�鲽�裺��ȡ��Ʒm gװ������У���С�Թ��з���С�ʹ��������ӣ��ټ�������ϡ���ᣬ�ٰѽ��ҷŵ��Թ��У���500 mLϴƿ��װ��һ�����ı���̼��������Һ����С�Թ�С�ķ���ϴƿ����ͼ2�����Ǻ�ϴƿ�ĸ��ӣ����ڴ����������ϣ������ܽ⣬����Ƭ���������ã���Ӧ�ӽ���ɣ���������ֱ��ϴƿ����Һ������������Ͳ��ȡϴƿ��������Һ�����ΪV mL��ʹ�ý��ҵ�Ŀ����_______�������ӵ�������_______������̼�����ƴ���ˮ�����Է�ֹ_______����֪���¶���CO2�ܶ����� g/mL����άC����Ƭ��NaHCO3������_______��
��4�����в������ܵ��²ⶨ���ƫС����_______��
a��С�Թ������������ϴƿ�� b��ʵ�������������Ͳ����
c������m gάC����Ƭʱ��������������ȷ��������ƽָ��ƫ��������
d��άC����Ƭ��װ�������ʱ��������
��5��ijͬѧ���װ�ã���ͼ3���ⶨNaHCO3������ʵ����Ʒ��ϡ���ᷴӦ�IJ�����_______��
���ⶨNaHCO3������Ҫ�����������_______��_______��
a����Ʒ����m g
b����Ӧǰ��Aװ������a g��a1 g
c����Ӧǰ��Bװ������b g��b1 g
d����Ӧǰ��Cװ������c g��c1 g
��ѡ������һ�����ݣ���ʾNaHCO3����_______��ָ����װ�õ�һ��ȱ����_______��

���𰸡� ���Ǻ�ά����C 3CO2 ��ֹάC����Ƭ��NaHCO3�������ᷴӦ����CO2�ݳ� ʹ��Ӧ��� CO2�ܽ� 21��v/11m bd ��Y����������б��ʹ����������Ʒһ�� ab �������10��ac ![]() ��
��![]() ���ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ����
���ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ����
����������������Ϣ֪����1��άC����Ƭ�������л�����г�ζ���ϡ������ᡢ���Ǻ�ά����C����2����άC����ƬͶ��ˮ�У��۲쵽�д������ݲ�������Ҫ����Ϊ�����ᣨH3C6H5O7����NaHCO3������Ӧ���÷�Ӧ��3NaHCO3+H3C6H5O7= Na3C6H5O7+3H2O+ 3CO2 ������3���ⶨNaHCO3������ʹ�ý��ҵ�Ŀ����. ��ֹάC����Ƭ��NaHCO3�������ᷴӦ����CO2�ݳ��������ӵ�������ʹ��Ӧ��֡�����̼�����ƴ���ˮ�����Է�ֹCO2�ܽ⡣��֪���¶���CO2�ܶ����� g/mL����άC����Ƭ��NaHCO3�����ǡ����ɶ�����̼�����Ǧ�vg. NaHCO3��CO2,, ![]() ��
��![]() ,x��21��v/11g. ��4���������ܵ��²ⶨ���ƫС����b��ʵ�������������Ͳ����. d��άC����Ƭ��װ�������ʱ��������. ��5���ⶨNaHCO3������ʵ����Ʒ��ϡ���ᷴӦ�IJ����ǽ�Y����������б��ʹ����������Ʒһ�ˡ����ⶨNaHCO3������Ҫ�����������a����Ʒ����m g��b����Ӧǰ��Aװ������a g��a1 g��a����Ʒ����m g��c����Ӧǰ��Bװ������b g��b1 g����ѡ������һ�����ݣ���ʾNaHCO3������
,x��21��v/11g. ��4���������ܵ��²ⶨ���ƫС����b��ʵ�������������Ͳ����. d��άC����Ƭ��װ�������ʱ��������. ��5���ⶨNaHCO3������ʵ����Ʒ��ϡ���ᷴӦ�IJ����ǽ�Y����������б��ʹ����������Ʒһ�ˡ����ⶨNaHCO3������Ҫ�����������a����Ʒ����m g��b����Ӧǰ��Aװ������a g��a1 g��a����Ʒ����m g��c����Ӧǰ��Bװ������b g��b1 g����ѡ������һ�����ݣ���ʾNaHCO3������![]() ��
��![]() �����ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ���ա�
�����ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ���ա�
��


 �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼװ�ã��ش������й����⣺
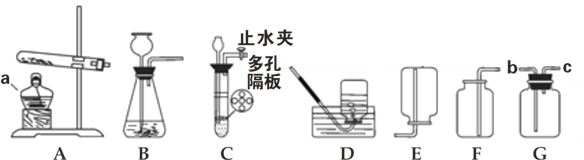
��1��װ��ͼ������a������Ϊ___________��
��2�����ü�������غͶ������̻����ķ�������ȡ���ռ���������ѡ�õ�װ����__________����Ӧ�Ļ�ѧ����ʽ��_______________________________��
��3������Gװ�ò����ſ������ռ�����,����Ӧ��_________��ѡ��b����c�����˽���
��4����ȡ������̼���ѡ�õķ���װ����____________����װ������һ����ѡ�õ�װ����ȣ�����Ҫ�ŵ���______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڴ�������������ȷ����
A����Ӧǰ����������� B���ܼӿ컯ѧ��Ӧ����
C����Ӧǰ������ʲ��� D����ʹ�κ����ʼ䶼������ѧ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˲ⶨ��ƷCaSO4XH2O��CaCO3��������ɣ�ʵ��С��������ͼ��ʾ��װ�ã��г�����ʡ�ԣ�����ʵ�顣

��1��ʵ��ǰ����Ҫ_________����װ����Ʒ��װ��A�м�ʯ�ҵ�������___________��
��2��Cװ�õ�������Ӧ����Ũ�������___________�����ʡ�
��3����֪CaSO4XH2Oʧȥȫ���ᾧˮ���¶�Ϊ160�棬1350��ʱCaSO4��ʼ�ֽ⣻CaCO3��900��ʱ�ֽ���ȫ���ֿ���Bװ���¶�900�����ʵ�飬���ɼ�������ʵ�����ݣ�
a����Ʒ������Ϊm1g b����Ӧ�������й��������Ϊm2g
c��װ��Cʵ�������m3g d��װ��Dʵ�������m4g
��ʵ����m1=22.20 g��m3=3.60 g��m4=2.20 g��
����Ʒ��CaCO3������___________ g����X��ֵ_____________��
��4��װ��E��������_________����ȱ��Eװ�ã����CaCO3������___________��ѡ�ƫ����ƫС���������䡱����X��ֵ��___________��ѡ�ƫ����ƫС���������䡱����
��5������Ϊ����ѡ������_________��ѡ����ţ��������Ҳ�����X��ֵ��
A��ab B��abd C��bcd
��6��CaSO4���¶ȴﵽ1350��ʱ�ֽ�����һ�ֹ������������������壬����һ�������д̼�����ζ����ֽ�Ļ�ѧ����ʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������ѧϰ��ѧ����Ҫ���ߡ�
��1���û�ѧ���ű�ʾ��
��2���ԭ��______________��
��笠�����____________��
��3����������____________��
���ܱ�����ֱ���������õ�����________��
��2������H��O��C��Na����Ԫ�أ���ѡ�����е�Ԫ��д���������ʵĻ�ѧʽ��
�������˹���������_________��
�ں����Ƽ�Ƶõġ��_________��
��������ȼ��_______________��
���ܹ�������������_______________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��һ����̼���»�ԭ������_____________��
����˿��������ȼ��____________��
��ϡ���������_______________��
������β����NO��CO�ڴ������������ɿ���������������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڡ���ѧ���������ʶ����ȷ���ǣ�������
A. �ú�С�մ�ķ��ͷ۱��Ƹ��
B. ���մ����Ͼɵ�ؼȿɽ�Լ������Դ�ֿɼ��ٻ�����Ⱦ
C. �þ�����ϩ������ʳƷ��װ���к�����
D. Ϊ���ţ�̵ĺ�����������ţ�������������谷
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������£�һ���ܱ������ڷ���ij��Ӧ����÷�Ӧǰ������ʵ��������±���ʾ��
����˵������ȷ����
���� | a | b | c | d |
��Ӧǰ������/g | 30 | 20 | 10 | 15 |
��Ӧ�������/g | x | y | 0 | 10 |
A. �μӷ�Ӧ��c��d����֮����2:1 B. x��ȡֵ��Χ�ǣ�0��x��30
C. ��y��20ʱ���÷�Ӧ�ǻ��Ϸ�Ӧ D. x+y=65
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�����˵����ȷ���ǣ� ��
A. ���ӿ����ٷ֣���ԭ�Ӳ����ٷ� B. ���Ӵ�ԭ��С
C. ԭ��ʧȥ���ӱ�������� D. ���ӡ�ԭ�ӡ����Ӷ����Թ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com