��2013?����ģ�⣩�������ƣ� Na
2O
2����һ�ֻ�ɫ���壮һ�οƼ���У�ij��ѧ��ȤС���ͬѧ������Na
20
2�������������ͼ1��ʾ�ձ��У���������C0
2���ִ��������������Ϩ������ĺ�Ϩ��ͬʱҲ����ط�����ȼ��������
I��ʵ������С������������Ϩ������ĺ�Ϩ�𡱵�����˵��������̼����
�ܶȱȿ�����ȼ�ա���֧��ȼ��
�ܶȱȿ�����ȼ�ա���֧��ȼ��
�����ʣ�
����Ϊʲô��ȼ���أ�С��ͬѧ����������ȼ�յ�������ָ����Ҫȼ�գ��������㡰���ǿ�ȼ���һ����������Ҫ�����������
����������������Ӵ����¶ȴﵽ�Ż�㣨��ﵽȼ������Ҫ������¶ȣ�
����������������Ӵ����¶ȴﵽ�Ż�㣨��ﵽȼ������Ҫ������¶ȣ�
��
������⣺��ʵ�����������θ���ȼ���ṩ�����������أ�
�������ϣ��ڳ�����Na
2O
2��CO
2���ܷ�����ѧ��Ӧ��������DZˮͧ������������
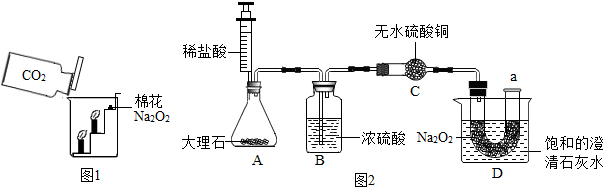
���ʵ�飺�������ۺ�С��ͬѧ�������ͼ2��ʾ��ʵ�����̽����
ʵ�鲽�裺
��1������ͼ���Ӻ�װ�ã�������������ԣ�
��2���ٰ���ص��Լ����˶�Ӧ�������У�
��3���ƶ�ע�����Ļ�������ϡ����ע����ƿ�ڣ����̶�����λ�ã�
��4��һ��ʱ�����a������һ�������ǵ�Сľ�����۲�����
ʵ������
��l���������û�г�����ɫ��
��2�������ǵ�Сľ����ȼ��
��3�����͵ij���ʯ��ˮ����ǣ�
ʵ�������
��1��Dװ�õ��ձ��ڱ���ǵ�ԭ����
Na2O2��CO2��Ӧ�ų�������ʹ�������Ƶ��ܽ�ȼ�С�������˳���
Na2O2��CO2��Ӧ�ų�������ʹ�������Ƶ��ܽ�ȼ�С�������˳���
��
��2��Cװ�õ�������
����ˮ�����Ƿ���
����ˮ�����Ƿ���
��
ʵ����ۣ�
��l����Сľ����ȼ��˵����Na
2O
2��CO
2��Ӧ������
��������O2��
��������O2��
��
��2�������͵ij���ʯ��ˮ����ǡ�˵��Na
2O
2��CO
2��Ӧ
�ų�
�ų�
������ա��ų������������Ӷ�ʹ��������ȼ��������




