
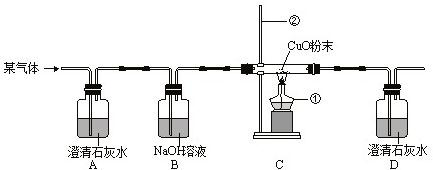
ij�����п��ܺ���H2��CO��CO2�е�һ�ֻ��֣�Ϊȷ��������ijɷ֣�ijͬѧ��������µ�ʵ��װ�ã�

��1��д��������������ƣ���________________����________________��
��2�����ܹ۲쵽______________________________________����֤���������к���CO2��
��3�����������к���H2����C���ܹ۲쵽��������_______________________________��
��4��B��NaOH��������______________________________________________________��
��5����֤��CO���ڵĹ������漰���ķ�Ӧ�Ļ�ѧ����ʽΪ��
________________________________________________________________________��
��6��������λͬѧ����ƽ���ʵ���Ƿ�һ�����ж�H2�Ĵ��ڣ�________����ǡ���
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
ij�����п��ܺ���H2��CO��CH4��Ϊ�ж�����ɣ���������ʵ�飺��ȼ�������壬��������Ϸ���һ��������С�ձ���������ˮ�����ɣ����ձ�Ѹ�ٵ�����ע����������ʯ��ˮ��ʯ��ˮ����ǣ�������������һ�ֻ�����ɵģ��������Ŀ��������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007���Ϻ��г��л�ѧ��ѧ���������Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com