

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ѧ������ ���ͣ�013
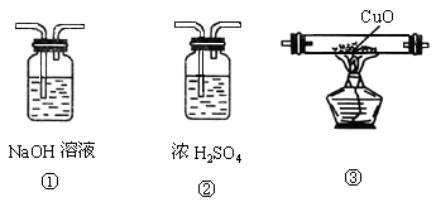
A���٢ڢ��������� B���٢ۢ��������� C���ۢ٢��������� D���ڢ٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���¿α���������˼ά�˽̰滯ѧ���꼶�ϲ� ���ͣ�058
��
���л�������������̼��һ����̼���壬ijͬѧ���ô����������һ����̼��ԭ����ͭ����֤��Ӧ����������ͼ��ʾװ����գ�
(����A����ͼ��ʾʢ��CO2��CO������������ƿ��Ũ���������ˮ��)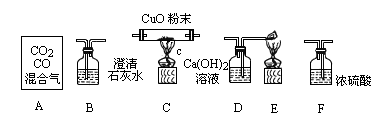
(1)������ĸ��ű�ʾװ�����ӵ���ȷ˳��(ÿ��װ��ֻ����һ��)��A��________________��
(2)Cװ���й۲쵽��������________��Eװ���й۲쵽��������________________��
(3)Bװ�õ�������________________����Ӧ�Ļ�ѧ����ʽΪ________________��
(4)Fװ�õ�������________________��
(5)д������װ���з����Ļ��Ϸ�Ӧ�Ļ�ѧ����ʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ����Ԫ��ϰ�������꼶��ѧ������ ���ͣ�058
��
���л�������������̼��һ����̼���壬ijͬѧ���ô����������һ����̼��ԭ����ͭ������֤��Ӧ����������ͼ��ʾ��ʵ��װ�ûش�����
(����A����ͼ��ʾʢ��CO2��CO������������ƿ)��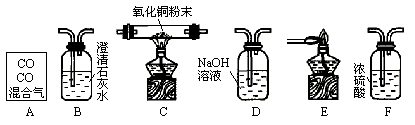
(1)������ĸ��ű�ʾװ�����ӵ���ȷ˳��(ÿ��װ��ֻ����һ��)��A��________��
(2)Cװ���й۲쵽��������________��
(3)Bװ�õ�������________����Ӧ�Ļ�ѧ����ʽΪ��________��
(4)Dװ�õ�������________��
(5)д������װ���з������Ϸ�Ӧ�Ļ�ѧ����ʽ��________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com