
 �ڻ��������У�ԭ����������ȫ��ת���ɲ�Ʒ��ijУѧ���������Ĺ�ҵ�����ռ�����ʳɷֽ����о������Dz������ϵ�֪���ڹ�ҵ����ʳ�κ�ˮ��ɱ���ʳ��ˮ��ͨ����⣨ͨ�磩�Ƶ��ռ���Һ���ٽ���ҺŨ�����ɵõ������ռͨ������л��ɵõ�������������Cl2���������Ʋ�ù�ҵ�����ռ��п��ܺ���̼���ơ��Ȼ��ƣ��������ʵ������֤���裮
�ڻ��������У�ԭ����������ȫ��ת���ɲ�Ʒ��ijУѧ���������Ĺ�ҵ�����ռ�����ʳɷֽ����о������Dz������ϵ�֪���ڹ�ҵ����ʳ�κ�ˮ��ɱ���ʳ��ˮ��ͨ����⣨ͨ�磩�Ƶ��ռ���Һ���ٽ���ҺŨ�����ɵõ������ռͨ������л��ɵõ�������������Cl2���������Ʋ�ù�ҵ�����ռ��п��ܺ���̼���ơ��Ȼ��ƣ��������ʵ������֤���裮| ʵ�鲽�� | ʵ������ | �� �� |
| ��ȡ������������ˮ | ������ȫ�ܽ� | -- |
| �����Թ�ȡ������Һ���������ϡ���ᣬ���ϴ����ܵĵ������������ܵ���һ�˲��� |
�� �� |
֤��ԭ�����ռ��к���̼���� |
| �����Թ����ټ��������� | �а�ɫ�������� | ֤��ԭ�����ռ��к��� |
| ʵ�鲽�� | ʵ������ | �� �� |
| �ڳ���ʯ��ˮ | ���� ��ɫ���� |
|
| �� | ֤ �Ȼ��� |
| ||
| ||
| ||
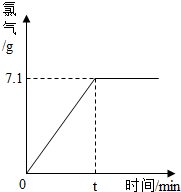
| 117 |
| 71 |
| x |
| 7.1g |
| 71 |
| 2 |
| 7.1g |
| y |
| 71 |
| 80 |
| 7.1g |
| z |
| 8.0g |
| 100g-7.1g-0.2g |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?��ͽ��ģ�⣩ijУѧ���������Ĺ�ҵ�����ռ�����ʳɷֽ����о���
��2013?��ͽ��ģ�⣩ijУѧ���������Ĺ�ҵ�����ռ�����ʳɷֽ����о���| ʵ�鲽�� | ʵ������ | ���� |
| ��ȡ������������ˮ | ������ȫ�ܽ� | û�в��������� û�в��������� |
| �����Թ�ȡ������Һ��������������ϴ����ܵĵ������������ܵ���һ�˲���ʢ�� ����ʯ��ˮ�� ����ʯ��ˮ�� �ձ��� |
����ʯ��ˮ����� ����ʯ��ˮ����� |
ԭ���������к��� |
| �������Թ��м��� | �а�ɫ�������� | ԭ�����ռ��к��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧ����ʽ | |
| �Լ�1 | |
| �Լ�2 | |
| �Լ�3 |
| ʵ�鲽�� | ʵ������ |
| ��1���ѹ����ռ�����ˮ | |
| ��2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com