
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ��/g | Ca��OH��2 | 0.19 | 0.17[ | 0.14 | 0.12 | 0.09 | 0.08 |
| NaOH | 31 | 91 | 111 | 129 | 313 | 336 | |
���� ��1�����ݲ�������Һ��Ϊ������Һ��һ�㷽���ǣ��������ʣ������ܼ��������¶ȣ������Ca��OH��2���ܽ�����¶ȱ仯����������
��2�������ܽ�ȵĺ�����з������㣻
��3��CaO��ˮ��Ӧ�����������ƣ��ų���������Ca��OH��2���ܽ�����¶ȵ����߶���С������ϱ�����Һ���ʵ�������������ʽ�������
��4����Ca��OH��2��NaOH���ܽ�����¶�Ӱ����������ᴿNaOH����ķ�����
��5�����ݽ�pH��ֽ������ˮ��ʪ���ٽ��вⶨ����������Һ��pHֵ����ʹ������������Һ�ļ��Ա������н��
��� �⣺��1��Ca��OH��2���ܽ�����¶ȵ����߶���С������Ҫʹ�䲻������Һ��Ϊ������Һ�ɲ�ȡ�������ʡ������ܼ������µķ�������ʯ������ˮ��ˮ��Ӧ�ҷ���ʹ��Һ�¶����ߣ����ܽ�ȼ�С��ͬʱ�����������ƣ�Ҳ��ʹ��������Һ��Ϊ������Һ������ʩ��ȷ���ǣ��٢ڢݢޣ�
��2��������20��ʱ��NaOH���ܽ����91g��������20��ʱ��10gˮ����ܽ�9.1gNaOH�����ܽ�ȵĺ����֪����191g������Һ����10gˮ���ٽ��µ�20�棬������NaOH���������Ϊ9.1g��
��3��CaO��ˮ��Ӧ�����������ƣ��ų���������Ca��OH��2���ܽ�����¶ȵ����߶���С��������Һ���ʵ���������=$\frac{�ܽ��}{�ܽ��+100g}$��100%�����ܽ��Խ����������Ҳ��Խ�����Լ���һ����CaO��õ�����Һ������Һ�����ܽ��С�ڼ���Һ����ʱ��Һ������������ϵ���ң��ף�
��4��Ca��OH��2���ܽ�����¶ȵ����߶���С��NaOH���ܽ�����¶ȵ����߶���������Ҫ��60��ʱ��Ca��OH��2��NaOH�������ʵı�����Һ�еõ��ϴ�����NaOH���壬Ӧ��ȡ���½ᾧ��Ȼ����˵�����������
��5����pH��ֽ������ˮ��ʪ���ٽ��вⶨ����������Һ��pHֵ����ʹ������������Һ�ļ��Ա������������������Һ��pHƫС��
�ʴ�Ϊ����1��D��
��2��9.1g��
��3������
��4�����½ᾧ��
��5��ƫС��
���� �˽�Ca��OH��2��NaOH�ܽ�ȵ��ܽ�����¶ȵı仯������ⶨ��ҺpHֵ����ȷ���������ܾ���ѧ֪ʶ��ȷ������𣬱������ڿ����֪ʶ�����պ�Ӧ�ã�����ѧ��������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
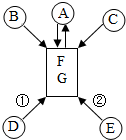 ��֪A��B��C��D��E��F��G�dz��л�ѧ������7�����ʣ���ˮ�к��д�����F��G������ת����ϵ��ͼ��ʾ��ʡ�Է�Ӧ�����Ͳ��ַ�Ӧ����������Ӧ���١���Ϊ�ֽⷴӦ������˵��������ǣ�������
��֪A��B��C��D��E��F��G�dz��л�ѧ������7�����ʣ���ˮ�к��д�����F��G������ת����ϵ��ͼ��ʾ��ʡ�Է�Ӧ�����Ͳ��ַ�Ӧ����������Ӧ���١���Ϊ�ֽⷴӦ������˵��������ǣ�������| A�� | ��DΪ��ɫ���壬��E����Ϊ��ɫ���� | |
| B�� | ��B�����ڿ�����ȼ�գ���C���ܿ��������ᷴӦ | |
| C�� | ��δ��������ˮ��F��G��������A | |
| D�� | ͨ������£�A�����ǹ�������壬����������Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ͼ��ʾ������ʵ��ļ���ƿ�ײ�Ԥ�Ⱦ�װ������ˮ��
ͼ��ʾ������ʵ��ļ���ƿ�ײ�Ԥ�Ⱦ�װ������ˮ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij�������ϡ���ᣬ��������ɫ���壬��ù���һ���ǽ������� | |
| B�� | ʹ��̪��Һ����ɫ����Һһ����ʹʯ����Һ����ɫ | |
| C�� | ϡ��Ũ����ʱ��Ũ��������ע��ʢˮ����Ͳ�� | |
| D�� | ��ʯ��ˮ��ͨ�������̼����Һ��PH����С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2017���п��ڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ѡ�������
2017��11��15�չ���Ժ�������ǿ�����ٿ�����Ժ������飬����ͨ���ˡ���ʮ���塱��̬���������滮�����������������ڻ�����������
A. �����սոѣ��������� B. ������ˮֱ���ŷź���
C. ����ʹ��һ���Կ��ӣ����ϴ��� D. ����������١���ɫ��Ⱦ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com