ijУ�����п���ѧʵ�鿼�飬���������������⣺����Һ�����ƣ��ڶ�����̼����ȡ���ռ�������������ȡ���ռ�������涨��ѧ����ǩȷ�����⣮
I��ͬѧ��ǩ���ʦ��������������������ҩƷ��ʵ��̨ǰ��
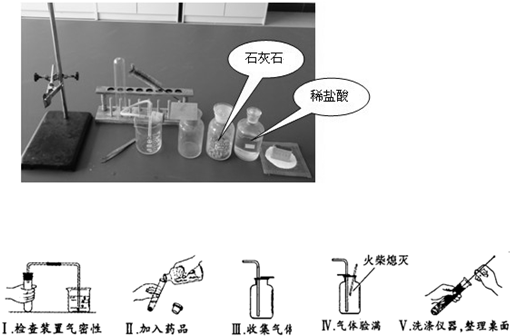
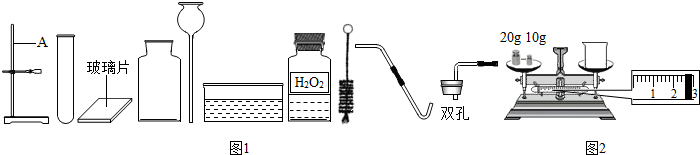
��1����ͼ������A��������
����̨
����̨
����ͬѧ�鵽�Ŀ�����
��
��
������ţ���ʵ��ǰ��ͬѧ��������ȱ����һ��ҩƷ����ҩƷ��
��������
��������
��
��2������ѡҩƷ��Ҳ�������һ������������Ʊ������еĻ�ѧ����ʽΪ
CaCO3+2HCl�TCaCl2+CO2��+H20
CaCO3+2HCl�TCaCl2+CO2��+H20
��
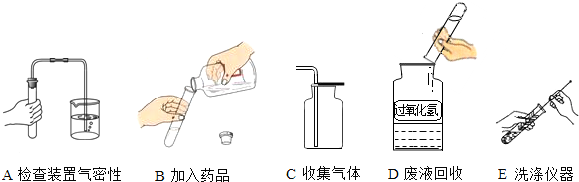
II��ͬѧ��ǩ�����ʵ����ʦҪ���ش��������⣬��������ش�
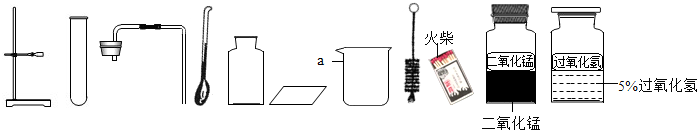
��3���������ʳ���к�������̼���ƣ��ᴿ�ķ�����
�������������������ݣ������ᾧ����
�������������������ݣ������ᾧ����
��
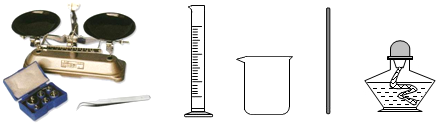
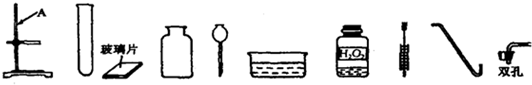
��4����Ҫ���ᴿ��Ĺ���ʳ������50g������������Ϊ6%��ʳ����Һ����Ҫ���������ĸ��������裺
��ͨ�����㣬��������ʳ����Һ��Ҫ�����Ȼ���3g��ˮ47g����47mL����
�ڽ�������ƽ����ƽ��ѹ����Ȼ���ֱ�ӷ��������ϳ�����Ȼ�����ձ��У�
������Ͳ��ȡ47mLˮ������ʢ���Ȼ��Ƶ��ձ��У��ò���������ʹ��ȫ���ܽ⣻
�ܽ����ƺõ���Һװ���Լ�ƿ�У��Ǻ�ƿ�������ñ�ǩ��
������ʵ����������У��������Բ���ʧ��IJ�����
��
��
������� ����