
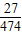 =0.019g��0.019g��0.004g�������˰�ȫ��������
=0.019g��0.019g��0.004g�������˰�ȫ��������


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���о����֣���Ԫ�������˵���ϸ�����Ӷ���������ϵͳ���裬��������մ����������������֯�涨��ÿ�¶���Ԫ�ص�������Ӧ������0.004 g���¡��������ҹ���������������ʳƷ֮һ��100 g����Լ��0.474 g������KAl(SO4)2·12H2O�����ʣ�
(1)��������Է�������Ϊ________________________��
(2)����һ��ʳ��100 g�������㣬��Ԫ�ص��������Ƿ���ȫ��������
(3)���ճ������У������ٳ������⣬��Ӧ��ע����Щ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������8�� ʳƷ�е��л������2010�굥Ԫ���Ծ���11���������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com