
| ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� |
| ���ǵ�����/g | 10 | 20 | 30 | 40 |
| ����CO2������/g | 3.52 | 7.04 | 8.8 | m |

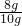 ��100%=80%
��100%=80%

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� |
| ���ǵ�����/g | 10 | 20 | 30 | 40 |
| ����CO2������/g | 3.52 | 7.04 | 8.8 | m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С���Ӻ�����һЩǶ��ɳ���ı��ǣ���Ҫ�ɷ�Ϊ̼��ƣ���Ϊ�ⶨ̼��Ƶĺ���������������ʵ�飺���ȳ�ȡ50 g������Ʒ��Ȼ��150 mL�������5�μ��루�����������ʾ��������ᷴӦ����ʵ������е����ݼ�¼���£�
| ϡ���� | ��һ��30 mL | �ڶ���30 mL | ������30 mL | ���Ĵ�30 mL | �����30 mL |
| ʣ��� | 40.0 g | X | 20.0 g | 10.0 g | 5.0 g |
��1��X = g��
��2��С����ñ�����Ʒ��̼��Ƶ����������� ��
��3�����Ѷ�����̼�ռ����������ж���g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С���Ӻ�����һЩǶ��ɳ���ı��ǣ���Ҫ�ɷ�Ϊ̼��ƣ���Ϊ�ⶨ̼��Ƶĺ���������������ʵ�飺���ȳ�ȡ50 g������Ʒ��Ȼ��150 mL�������5�μ��루�����������ʾ��������ᷴӦ����ʵ������е����ݼ�¼���£�
| ϡ���� | ��һ��30 mL | �ڶ���30 mL | ������30 mL | ���Ĵ�30 mL | �����30 mL |
| ʣ��� | 40.0 g | X | 20.0 g | 10.0 g | 5.0 g |
��1��X = g��
��2��С����ñ�����Ʒ��̼��Ƶ����������� ��
��3�����Ѷ�����̼�ռ�����������_____________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ǰ����ѧ���꼶���ϣ�������ϰ��ѧ�Ծ���12�·ݣ��������棩 ���ͣ������
| ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� |
| ���ǵ�����/g | 10 | 20 | 30 | 40 |
| ����CO2������/g | 3.52 | 7.04 | 8.8 | m |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com