


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
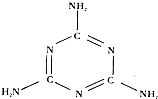 ���µ�ţ�� ���ұ��У������ʵĺ�����ÿ100�˲����� 2.95%���͵�2.8%�������谷�¼�֮���Ա�����������Ϊ��ȥ��ţ�̱�ǿ�������ʺ�����Ҫ��̫���ˣ�����ũ����ţ�̴ﲻ��Ҫ�Ų�ϧ�������գ������谷�Ļ�ѧʽΪC3H6N6���ṹ��ͼ������һ����;�㷺�Ļ���ԭ�ϣ�������Ϊ����ɫ���壬��ζ��������ˮ��������ˮ��ˮ��Һ�������ԣ�һ������½��ȶ������ڸ������ֽܷ�ų��ж����軯�
���µ�ţ�� ���ұ��У������ʵĺ�����ÿ100�˲����� 2.95%���͵�2.8%�������谷�¼�֮���Ա�����������Ϊ��ȥ��ţ�̱�ǿ�������ʺ�����Ҫ��̫���ˣ�����ũ����ţ�̴ﲻ��Ҫ�Ų�ϧ�������գ������谷�Ļ�ѧʽΪC3H6N6���ṹ��ͼ������һ����;�㷺�Ļ���ԭ�ϣ�������Ϊ����ɫ���壬��ζ��������ˮ��������ˮ��ˮ��Һ�������ԣ�һ������½��ȶ������ڸ������ֽܷ�ų��ж����軯��鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com