�⣺��1����98%��Ũ���������=
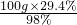
=30g��
��2��ϡ�����ܽ������Ļ�ѧ����ʽ�ǣ�3H
2SO
4+Fe
2O
3�TFe
2��SO
4��
3+3H
2O��
��3��3H
2SO
4+Fe
2O
3�TFe
2��SO
4��
3+3H
2O
294 160
100g��29.4% x
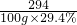
=
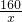
x=16g
��4���˳�����������������������=
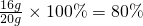
��5���跴Ӧ��������������Ϊy
Fe
2O
3��Fe
2��SO
4��
3160 400
16g y
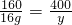
y=40g
��Һ����Ϊ��16g+100g+44g=160g
��������Һ�����ʵ���������=
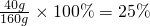
��6���������ֳ����������Ϊa����
2.8��96%=a��80%����1-20%����
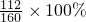
a=6���
�ʴ�Ϊ����1��30g��
��2��3H
2SO
4+Fe
2O
3�TFe
2��SO
4��
3+3H
2O��
��3��
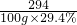
=
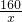
��
��4��80%��
��5��25%��
��6��6���
��������1��������Һʱ���������������伴���е�ʽ��⣻
��2�����������Ҫ�ɷ������������������������ᷴӦ���ɶ�Ӧ���κ�ˮ��
��3�����ݻ�ѧ��Ӧ����ʽ����μӷ�Ӧ��������ʱ�����뷽��ʽ����ı�������ȫ��Ӧ������������lOOg��29.4%��ϡ�����ڼ����DZ����������������ڴ��뷽��ʽ��
��4���������ij���ʵ���������=
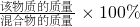
���ɣ�3�������������������������ʽ������⣻
��5������������ҺΪ��������Һ�����������ɸ��ݻ�ѧ����ʽ�������Һ����Ϊ��16g+100g+44g=160g���ݴ˿������������������
��6�����ݸֲ��к�������������ʯ�к���������ȿ���ʽ��⣮
�������������йػ�ѧ����ʽ����Һ�����������������ֵ��ۺϼ��㣬�����Ѷȴ����׳��������ݻ�ѧ����ʽ����ؼ����dz��л�ѧ���ص㣬�������ѵ㣬һ��ѧ������𣮱�����ѵ��ǣ�5����6��С�ʣ���5��С�⣬��Ӧ����Һ�����ʵ���������ʱ���ؼ���Ҫ�����ʵ���������Һ����������Һ������Ҫע�����в�����������������뽫��������������ȥ���籾����20g�ij��������ֻ��16g������������������Һ�У����Լ�����Һ�����DZ�����16g��������������20g���˴�ѧ�������׳�������6��С�⣬һ����Ŀ�г��ֺܶ����ݣ��е�ѧ���ͻ����ˣ���ʵ��ϸ���ⲻ�ѷ��֣������������ӹ���ʹ�ù����У���Ԫ�ص��������غ�ģ�ֻҪ����Ԫ�ص�������Ϊ���������г���ʽ��⣮

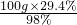 =30g��
=30g��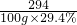 =
=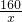
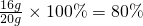
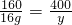
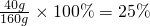
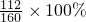
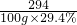 =
=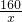 ��
��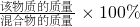 ���ɣ�3�������������������������ʽ������⣻
���ɣ�3�������������������������ʽ������⣻

