
����ѧ��ѧ֪ʶ����������⣺
��1���;��ϵ������ü���ϴ�ྫ��ˮ����ϴ����ԭ���� ____________ ____��
��2���������������Ԫ����50���֣����к�������Ԫ���� ����������ȱ��ijЩԪ��ʱ��Ӱ�콡�������¼���������ȱп��ص��� ��
A��ƶѪ B�����Ͳ� C��٪��֢ D����״���״�
��3����ҵ�ϲ��õ�ⱥ��ʳ��ˮ�Ʊ��������ƣ�2NaCl+2H2O ��� 2NaOH+Cl2��+H2������ʳ��ˮ�л���һ������NH4Cl���ڵ��ʱ������ը��Ϊ��ȥNH4Cl����ҵ�ϳ����õ���ļ��Է�Һ��Cl2��NH4Cl��Ӧ������Cl2��NH4Cl��ѧ�������ֱ�Ϊ3��2����ʹNH4Clת����N2���÷�Ӧ�Ļ�ѧ����ʽΪ�� ����˵���˷������ŵ�֮һ ��
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �����ʣ�g�� | ֬����g�� | ���g�� | �ף�mg�� | �ƣ�mg�� | ����mg�� |
| 39.2 | 17.4 | 5.0 | 570 | 320 | 5.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
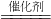 CH3OH����X�Ļ�ѧʽΪ________��
CH3OH����X�Ļ�ѧʽΪ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ֪ʶ�������������е���ϵ����������ѧ��ѧ֪ʶ����������⣺
��1��Ҫ����Ӳˮ����ˮ������ʹ�õ�������__________ _____����ҵ�����г��ù�¯��ˮ�����������¯�г���ʹ��Ӳˮ������ɹ�¯�ı�ը����ҵ�������� �������Խ���Ӳˮ��Ӳ�ȡ�
��2��Ҫ��ȥ��ˮ�е����������ʣ����Բ��õĻ���������________________��
��3��Ϊ��֤������ȫ�������δ�����IJ˽�ǰ������________________���飻
��4����ȼ��ȼ������ǰӦ����_______ _________��
��5������ij�ٻƽ����Σ�пͭ�Ͻ��Ƴɣ������� �ķ�����
 ��6�����پơ��к���һ�����ļ״�[CH3OH]�����ú��ʹ������Ѹ���½���ʧ����������������ҵ����ȡ�״��ķ���Ϊ��X + 2H2 ���� CH3OH����X�Ļ�ѧʽΪ ��
��6�����پơ��к���һ�����ļ״�[CH3OH]�����ú��ʹ������Ѹ���½���ʧ����������������ҵ����ȡ�״��ķ���Ϊ��X + 2H2 ���� CH3OH����X�Ļ�ѧʽΪ ��
��7��ú��һ�ֳ�����ȼ�ϣ���ͥ��ú�����˴�ʵ�ġ�ú�����˿ġ�����ú���ı仯�����ֱ仯���ŵ���___ _________ ____.��ʵ�ϣ�ֱ����ú��ȼ���Ƕ���Դ���˷ѣ�Ӧ�ᳫú���ۺ����ã����罫ú�ڸ�����ת����ˮú��(��Ҫ��һ����̼������)����д���÷�Ӧ�Ļ�ѧ����ʽ_____________ ____.
ú�������ڼ���ˮú������������������ŵ����壬Ŀ���� ________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ���е������о��꼶���ϣ��¿���ѧ�Ծ��������棩 ���ͣ������
 CH3OH����X�Ļ�ѧʽΪ ��
CH3OH����X�Ļ�ѧʽΪ ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com