某同学为探究某石灰石中碳酸钙的质量分数,该同学取25g石灰石放入烧杯中,然后加入146g稀盐酸,充分反应后,称得烧杯内物质的总质量为162.2g(石灰石中的杂质不参与反应,也不溶于水).
求:①产生CO2多少g?
②石灰石中碳酸钙的质量分数?
③稀盐酸中溶质的质量分数?
④反应后溶液中溶质的质量分数?
【答案】
分析:分析所发生的反应,可发现反应后总质量减轻是由于生成了气体二氧化碳,因此,可知恰好完全反应时放出二氧化碳的质量;然后利用二氧化碳的质量,根据反应的化学方程式,分别计算恰好完全反应时碳酸钙的质量和HCl的质量以及生产氯化钙的质量,最后使用质量分数的计算公式,求出石灰石中碳酸钙的质量分数、所用盐酸的质量分数和反应后溶液中溶质的质量分数.
解答:解:①反应中产生二氧化碳的质量为25g+146g-162.2g=8.8g;
②设参与反应的CaCO
3质量为x,稀盐酸中溶质的质量为y,生产氯化钙的质量为z.
CaCO
3+2HCl=CaCl
2+H
2O+CO
2↑
100 73 111 44
x y z 8.8g
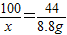
x=20g
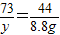
y=14.6g
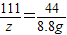
z=22.2g
石灰石中碳酸钙的质量分数为:
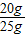
×100%=80%.
③稀盐酸中溶质的质量分数=

×100%=10%.
④反应后溶液中溶质的质量分数=
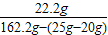
×100%=14.1%
故答案:①产生CO
2为8.8g;
②石灰石中碳酸钙的质量分数为20%;
③稀盐酸中溶质的质量分数为10%;
④反应后溶液中溶质的质量分数为14.1%.
点评:本题主要考查学生运用化学方程式和质量分数公式进行计算的能力.增加了学生分析问题的思维跨度,强调了学生整合知识的能力.

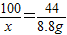
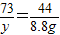
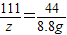
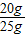 ×100%=80%.
×100%=80%. ×100%=10%.
×100%=10%.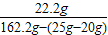 ×100%=14.1%
×100%=14.1%
