

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ���ʵ�飬��Э�������ʵ�鱨�棬���ش�������⣮
��һ��ʵ��Ŀ�ģ��о���ѧ��Ӧ�����뷴Ӧ��Ũ�ȵĹ�ϵ
������ʵ����Ʒ��ϡ����NaOH��Һ��Ƭ��ɫʯ����ֽ��ɫʯ����ֽ�ձ���������ͷ�ι�
������ʵ�����ݣ�
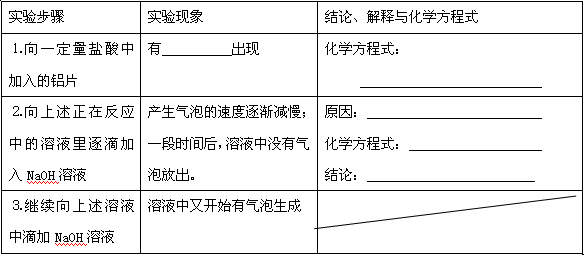
| ʵ�鲽�� | ʵ������ | ���ۡ������뻯ѧ����ʽ |
| 1����һ�����������м�����Ƭ | ��_____________���� | ��ѧ����ʽ�� _____________________ |
| 2�����������ڷ�Ӧ�е���Һ����μ���NaOH��Һ | �������ݵ��ٶ�������һ��ʱ�����Һ��û�����ݷų��� | ԭ��_______________ ��ѧ����ʽ��__________ ���ۣ�_______________ |
| 3��������������Һ�еμ�NaOH��Һ | ��Һ���ֿ�ʼ���������� | ------------------ |
���ģ���ͬѧ��ʵ�鲽��������������̽����
��1���������Ϻ��֪����Ҳ�ܺ�����������Һ��Ӧ2Al+2NaOH+2H2O�T2NaAlO2+3H2��Ϊ֤������������ʱ��NaOH�Ѿ���ȫ��������ͬѧ���Բ���______�IJ���
��2����������Ƭ��ȫ�ܽ⣬��õ���H2����Ƭȫ�������ᷴӦ�õ���H2������______��
A��ƫ��B�����
C��ƫС��D����ȷ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������7�� Ӧ�ù㷺���ᡢ��Ρ�2010�굥Ԫ���Ծ���2���������棩 ���ͣ������
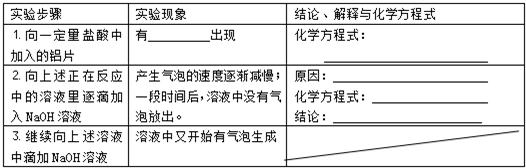
| ʵ�鲽�� | ʵ������ | ���ۡ������뻯ѧ����ʽ |
| 1����һ�����������м�����Ƭ | ��_____________���� | ��ѧ����ʽ�� _____________________ |
| 2�����������ڷ�Ӧ�е���Һ����μ���NaOH��Һ | �������ݵ��ٶ�������һ��ʱ�����Һ��û�����ݷų��� | ԭ��_______________ ��ѧ����ʽ��__________ ���ۣ�_______________ |
| 3��������������Һ�еμ�NaOH��Һ | ��Һ���ֿ�ʼ���������� | ------------------ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com