
 ������ѩ�����İ�װ���ڳ�����һС�����������������ϵIJ���������ͼ������ϸ�Ķ����ش��������⣺
������ѩ�����İ�װ���ڳ�����һС�����������������ϵIJ���������ͼ������ϸ�Ķ����ش��������⣺


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
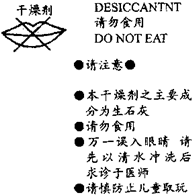 ����ʳƷ��˾�ڡ�����ѩ�����İ�װ���ڶ�����һС�������������IJ������ּ���ͼ���뿴ͼ�ش��������⣺
����ʳƷ��˾�ڡ�����ѩ�����İ�װ���ڶ�����һС�������������IJ������ּ���ͼ���뿴ͼ�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ����� DESICCANT ����ʳ�� DO NOT EAT ��ע�� �����������Ҫ�ɷ�Ϊ��ʯ�ң�CaO�� ��һ�����۾�����������ˮ��ϴ��������ҽʦ ��������ͯȡ�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ����� �Ķ�������Ϣ���ش��й����� ������ѩ�����İ�װ���ڳ�����һ�����������������ϵIJ���������ͼ��ʾ��  ��1����ʯ���������������д���йػ�ѧ����ʽ�� CaO+H2O�TCa��OH��2 CaO+H2O�TCa��OH��2 ����2������ʳ������Ϊ�����ʳ�ú�������� ��ʴ ��ʴ ���ã��Ҹø��������ˮ����� �� �ȣ���3�������У��ʼ����ڽ��к�������ʱ��ͨ�������DZ���Ĵ�����С��϶����������̼��ʹ�������ʣ�Ϊ��ֹͣ�����ĺ������ôﵽ���ʵ�Ŀ�ģ����dz��Ѽ�������ʯ��ˮ���б��ʣ��仯ѧ��Ӧԭ���ɱ�ʾΪ��д����ѧ����ʽ�� CO2+Ca��OH��2�TCaCO3��+H2O CO2+Ca��OH��2�TCaCO3��+H2O ���鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |