��������������������Ӧ�ù㷺��
��1��������Ʒʹ�ý������ϵ���
������ĸ��ţ���

��2����Ȼ���еĽ�������Ի�������ʽ���ڣ���ȡ�������ʵķ����ж��֣���ҵ���ó�����ʯұ�����Ļ�ѧ����ʽΪ
������ұ�������л�ԭ��������ұ������õ�ⷨ��ͨ��ֽ����ڵ���������ȡ���Ļ�ѧ����ʽΪ
��
��3����ϧ�ͱ���������Դ�Ĵ�ʩ֮һ�Ƿ�ֹ������ʴ��ʹ�ù��IJ˵���ϴ����Ҫ��ʱ���ɷ��ã����Լ���
������ʴ��Ӱ�죮
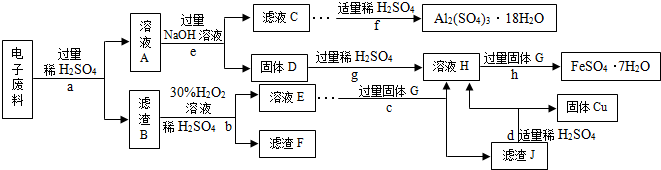
��4�����շϾɽ����DZ���������Դ����Ҫ��ʩ����ѧС���ͬѧ�ӵ��������л���˺�Cu 70%��Al 25%��Fe 4%������Au��Pt�ĵ��ӷ��ϣ��������»��մ�����ʡ�Է�������Ͳ��ֲ�Ʒ�����������������Ʒ����������Ԫ�أ����ش��й����⣺
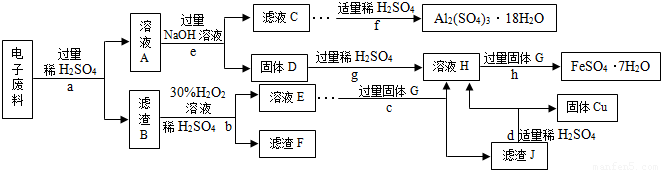
�ٲ���c�ڼ������GǰӦ�ȳ�ȥ��Һ�еĹ������⣬��ȥ��������ļ����û�ѧ����ʽ��ʾΪ
��
����ҺA�к��е�������
��
��A��J���������У�������Ԫ�ص���
������ĸ��ţ���



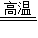 3CO2+2Fe��ͨ��ֽ����ڵ�����������������������Ӧ�Ļ�ѧ����ʽΪ2Al2O3
3CO2+2Fe��ͨ��ֽ����ڵ�����������������������Ӧ�Ļ�ѧ����ʽΪ2Al2O3  4Al+3O2�������3CO+Fe2O3
4Al+3O2�������3CO+Fe2O3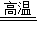 3CO2+2Fe��2Al2O3
3CO2+2Fe��2Al2O3  4Al+3O2����
4Al+3O2����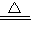 2H2O+O2�������2H2O2
2H2O+O2�������2H2O2 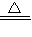 2H2O+O2����
2H2O+O2����