


 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ˮ�����/ml | �ƾ������/ml | ���ǰ�����/ml | ��Ϻ������/ml |
| 1 | 87.8 | 12.2 | 100 | 98.8 |
| 2 | 66.2 | 33.8 | 100 | 97.7 |
| 3 | 44.4 | 55.6 | 100 | 96.4 |
| 4 | 16.7 | 83.3 | 100 | 97.6 |
| 5 | 8.2 | 91.8 | 100 | 98.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ѧϰ�ܱ�����ѧ���˽̿α��п��桡2009��2010ѧ�ꡡ��4�ڡ���160�� �˽̿α��п��� ���ͣ�058
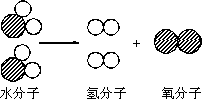
������ѧ֪ʶ�����ǿ��Դӡ����ˮʵ�顱�л�ø������Ϣ�����ۣ�װ����ͼ��ʾ���ش��������⣮
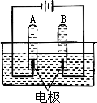
(1)A�Թ��ռ���������________���û���ȼ��������________��
(2)A�Թܲ����������B�Թܲ�����������������________��
(3)�÷�Ӧ�����ֱ���ʽΪ________��ͨ����ʵ��˵����ˮ����________��ɵģ�
(4)��ͼ��ˮ�ֽ����ʾ��ͼ���������Ĺ۵�������ˮ�ֽ�Ĺ���________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com