
������ɶ�ʵ������ȡ������̼��ʵ��̽����
A B C D F
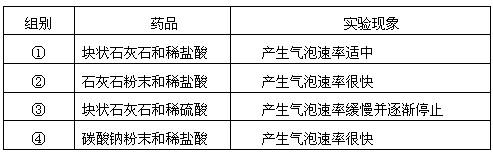
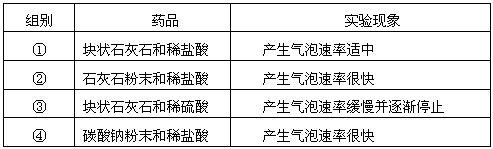
��ѡ��ҩƷ���±��Ƕ�����ҩƷ����ʵ��ļ�¼��
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ʯ��ʯ��ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
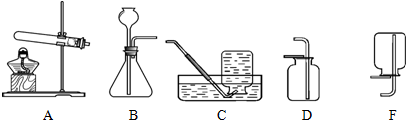
����ȡ���ռ��ĽǶȷ�����һ��ѡ��� ��ҩƷ������ĸ��ţ���ͬ��������ҩƷ������Ӧ�Ļ�ѧ����ʽΪ ��
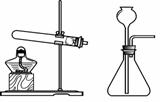
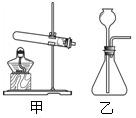
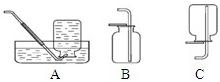
��ѡ��װ�á�ѡ�� ����װ�á�
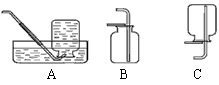
����ȡ���塣ѡ�� װ���ռ�������̼�������������� ��
��������顣�����ɵ�����ͨ����ɫʯ����Һ�У���Һ��죬ȷ���������Ƕ�����̼�����ּ��鷽���Ƿ���ȷ������ȷ��˵�����ɣ�������ȷ��д����ȷ�ļ��鷽���� ��
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ʯ��ʯ��ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
�鿴�𰸺ͽ���>>
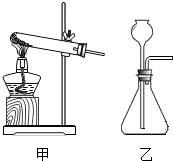
��Ŀ�����л�ѧ ��Դ����������ĩ�� ���ͣ�ʵ����
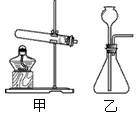
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ʯ��ʯ��ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
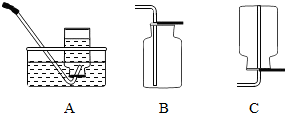
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com