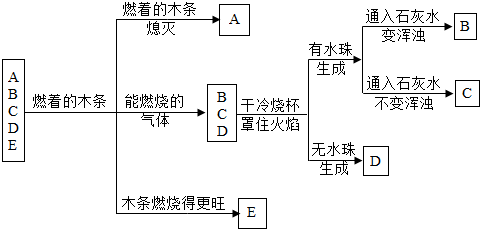
��A��B��C��D��E�������壬���Ƿֱ���������������һ����̼��������̼�������е�һ�֣�������һ��ȼ�ŵ�ľ���������飺A������ʹȼ�ŵ�ľ������Ϩ��B��C��D������ȼ�գ�E������ʹȼ�ŵ�ľ��ȼ�յø�����������ȼ�յ�B��C��D��������Ϸ��ֱ���һ����������ձ���B��C�����Ϸ����ձ�������ˮ����֣���D�����Ϸ����ձ�����û��ˮ�飬ȼ�պ�ֱ��������ձ���ע����������ʯ��ˮ����B��D�ձ���ʯ��ˮ����ǣ�C�ձ���û�б仯����ش��������⣮
��1��д��C��D��������Ļ�ѧʽ��
C________D________��
��2��B�����ڿ�����ȼ�յĻ�ѧ����ʽ��________��
��3��������Aͨ�����ʯ��ˮ�е�������________��
�÷�Ӧ�Ļ�ѧ����ʽ��________��
�⣺������֪������E������ʹȼ�ŵ�ľ��ȼ�յø����������ƶ�EΪ������
����Ϊ��Щ�����г������⣬��ȼ�յ�����Ϊ��������һ����̼�����飬�ʿ��ж�B��C��D����Ϊ������һ����̼�����飻��AΪ������̼���壬���ݡ�A������ʹȼ�ŵ�ľ������Ϩ��Ҳ���϶�����̼�����ʣ�
���ɡ�B��C�����Ϸ����ձ�������ˮ����֣���D�����Ϸ����ձ�����û��ˮ�顱��֪��B��CΪ�����ͼ��飨��Ϊˮ������Ԫ�أ�������������Ҳ����Ԫ�أ�һ����̼��û����Ԫ�أ�����ôD����һ����̼��
���ɡ�B��D�ձ���ʯ��ˮ����ǣ�C�ձ���û�б仯����֪��B��D��������ȼ�պ��ж�����̼�������ɣ���Cȼ�պ�û�ж�����̼�������ɣ��ʿ��ж�BΪ���飬CΪ��������Ϊ��������̼Ԫ�أ���
��B�����ڿ�����ȼ�յĻ�ѧ����ʽ�ǣ�CH
4+2O
2
CO
2+2H
2O��
����ΪA�Ƕ�����̼���壬����ʯ��ˮ��Ӧ����̼��Ƴ������ʰ�����Aͨ�����ʯ��ˮ�е������ǣ�ʯ��ˮ����ǣ��÷�Ӧ�Ļ�ѧ����ʽ�ǣ�Ca��OH��
2+CO
2=CaCO
3��+H
2O��
�ʴ�Ϊ����1��H
2��CO��
��2��CH
4+2O
2
CO
2+2H
2O��
��3��ʯ��ˮ����ǣ�Ca��OH��
2+CO
2=CaCO
3��+H
2O��
��������������֪������E������ʹȼ�ŵ�ľ��ȼ�յø����������ƶ�EΪ���������ݡ�A������ʹȼ�ŵ�ľ������Ϩ�𡱷��϶�����̼�����ʣ�Ȼ�������֪�������з����������ų����������ʣ����������壮
������������Ҫ�ǿ���ѧ�����ۺϷ�������������Ҫ��ѧ���߱��й�����Ļ���֪ʶ������Ҫ��ʵ������ľ����ͷ����������ѧʵ�����������������ʱ��Ҫ���������Ŀ��������������ϵʵ�ʣ���һ�����ƶϣ�

 CO2+2H2O��
CO2+2H2O�� CO2+2H2O��
CO2+2H2O��




 ��A��B��C��D��E���ֳ������ʣ���������ͼ��ʾ�Ĺ�ϵ����֪��C��һ�ֽ���������D����ʹ����ʯ��ˮ����ǣ�E�����ж������������ڵ�Ѫ�쵰��ϣ��ƶ�B���ʵĻ�ѧʽ��
��A��B��C��D��E���ֳ������ʣ���������ͼ��ʾ�Ĺ�ϵ����֪��C��һ�ֽ���������D����ʹ����ʯ��ˮ����ǣ�E�����ж������������ڵ�Ѫ�쵰��ϣ��ƶ�B���ʵĻ�ѧʽ��