��ѧ����������������أ��ճ������еĻ�ѧ֪ʶ�к࣮ܶ
��1����ʼ����ͭ��������ͭ�ɺס�������Ůͼ��10���������������ס�2011�������ᡱ��
������ʼ����ͭ��������ʱ������һЩͭ�⣬ͭ����Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu
2��OH��
2CO
3]��ͭ���������������ˮ�й��⣬�Ӽ�ʽ̼��ͭ�Ļ�ѧʽ��֪��������е�
������̼
������̼
�йأ�
����ͭ�ɺ���Ҫ�ɷ���ͭ�Ͻ�һ����˵���Ͻ���������Ĵ�������ȣ������ܻᷢ���ı䣬�ı�֮һ��
Ӳ��
Ӳ��
��
��������Ůͼ����Ҫ���Ϻ�����Ȼ��ά������Ȼ��ά�⣬�ִ����ǻ�ʹ�úϳ���ά��������Ȼ��ά��ϳ���ά���õĻ�ѧ������
ȼ��
ȼ��
����

��2�����գ���ý�屨��������ȼ�¼����е�����ݴ˵��������м����˲���ȫ�����Ӽ�������������ȼ���жϼ��������Ӽ��Dz���ѧ�ģ���Ϊ��ۺ��е���ҪӪ���ص��۱����ǿ���ȼ�յģ����۵Ļ�ѧʽΪ��C
6H
10O
5��n����ȫȼ�յIJ�����
ˮ�Ͷ�����̼
ˮ�Ͷ�����̼
��ȼ�չ����л����ŵ���ͷ���ս��ƵĴ̱���ζ���������������һ��Ӫ����ȼ�ղ����ģ���Ӫ������
������
������
����۲�������ȼ�գ�һ�������»��ᷢ����ը��2010��2���ҹ�ij������۳���װ���䷢����۱�ը������ش���Ա������Ϊ���������¼����������̿ɲ�ȡ�Ĵ�ʩ��
BC
BC
��
A���ܷ��Ŵ�����������
B���Ͻ����̣��ž���Դ
C��������ţ����ٷ۳�����
D����ߵ��۵��Ż��
��3������Ч��ת�������ɽ�����β�����ж����崦��Ϊ�����壬��ͼΪ�÷�Ӧ����ʾ��ͼ����ش��������⣺
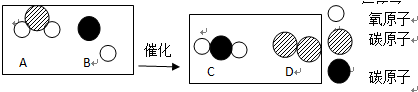
��ͼ����ʾ4�������У��������������
ABC
ABC
������ţ�
���ڷ�Ӧ�У�����C��D��������Ϊ
44��7
44��7
��
��4���١��������ܡ�������ͼ����һ�ֿ�������Я����С�;�����������װ����˿��ע���˻���̿���͵����֬�ȣ����л���̿��
����
����
���˵����ã�
��ũҵ�����������ʵ���������Ϊ10%��20%��NaCl��Һ��ѡ�֣��ֽ�300g 25%��NaCl��Һϡ��Ϊ15%��NaCl��Һ����Ҫ��ˮ������Ϊ
200
200
g��
�۸����±��ش����⣮
| �¶�/�� |
0 |
20 |
40 |
60 |
80 |
100 |
�ܽ��
/g |
NaCl |
35.7 |
36.0 |
36.6 |
37.3 |
38.4 |
39.8 |
| NH4Cl |
29.4 |
37.2 |
45.8 |
55.2 |
65.6 |
77.3 |
60��ʱ���������ֱ�ʢ��50g NaCl��NH
4Cl���ձ��У�������100g��ˮ������ܽ��Ϊ������Һ����
NaCl
NaCl
��Һ��




 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�





