
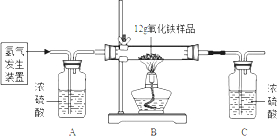
 ДіН¬С§ОӘІв¶Ё12gә¬ФУЦКөДСх»ҜМъСщЖ·ЦРСх»ҜМъөДЦКБҝ·ЦКэЈ¬АыУГПЎБтЛбәНРҝБЈЦЖИЎЗвЖшЈ¬ЙијЖБЛИзНјЛщКҫөДЧ°ЦГЈ¬ҪшРРУР№ШөДКөСйМҪҫҝЈЁМбКҫЈә3H2+Fe2O3
ДіН¬С§ОӘІв¶Ё12gә¬ФУЦКөДСх»ҜМъСщЖ·ЦРСх»ҜМъөДЦКБҝ·ЦКэЈ¬АыУГПЎБтЛбәНРҝБЈЦЖИЎЗвЖшЈ¬ЙијЖБЛИзНјЛщКҫөДЧ°ЦГЈ¬ҪшРРУР№ШөДКөСйМҪҫҝЈЁМбКҫЈә3H2+Fe2O3
| ||
| іЖБҝПоДҝ | ·ҙУҰЗ°ЦКБҝ | ·ҙУҰәуЦКБҝ |
| AПҙЖшЖҝәНЛщКўЕЁБтЛбөДЦКБҝ | 290Ј®3g | 291Ј®5 g |
| BІЈБ§№Ьј°ЛщКў№ММеөДЦКБҝ | 86Ј®3g | 83Ј®9g |
| CПҙЖшЖҝәНЛщКўЕЁБтЛбөДЦКБҝ | 284Ј®2g | 286.9g |
| ||
| 160 |
| x |
| 3ЎБ18 |
| 2.7g |
| 8g |
| 12g |



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
| AЎўЛ®өД·РМЪ |
| BЎў»Ж№ПЗРіЙұЎЖ¬ |
| CЎўМмИ»ЖшФЪИјЙХ |
| DЎўПгУН»У·ў |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
 Д©¶ЛҫЖҫ«өЖөДЧчУГКЗ
Д©¶ЛҫЖҫ«өЖөДЧчУГКЗІйҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
| AЎўіЈУГәПіЙІДБПОӘЛЬБПЎўәПіЙПрҪәЎўәПіЙПЛО¬ |
| BЎў»ҜКҜИјБПКЗЦёДҫМҝЎўЖыУНЎўХУЖш |
| CЎўОнцІМмЦ»КЗУЙУЪЖыіөОІЖшЕЕ·ЕФміЙөД |
| DЎўПтКіЖ·ЦРјУИлҙуБҝ·АёҜјБСУіӨұЈЦКЖЪ |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
| AЎў·ҙУҰОьИИЈ¬ҪөөНБЛҝЙИјОпөДЧЕ»рөг |
| BЎў·ҙУҰДЬ№»ҪөөНОВ¶ИЈ¬ҝЙИјОпІ»ТЧҙпөҪЧЕ»рөг |
| CЎўЙъіЙҙуБҝЛ®ХфЖшЈ¬ҪөөНҝЙИјОпЦЬО§СхЖшЕЁ¶И |
| DЎўЙъіЙСх»ҜВБёІёЗФЪҝЙИјОпұнГжЈ¬ёфҫшҝХЖш |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
| AЎўОпЦК¶јКЗУЙ·ЦЧУ№№іЙөД |
| BЎў·ЦЧУКЗұЈіЦДіР©ОпЦК»ҜС§РФЦКөДТ»ЦЦБЈЧУ |
| CЎў·ЦЧУКЗұЈіЦОпЦКОпАнРФЦКөДЧоРЎБЈЧУ |
| DЎў·ЦЧУКЗ№№іЙОпЦКөДТ»ЦЦБЈЧУ |
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com