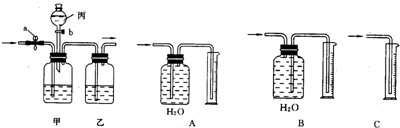
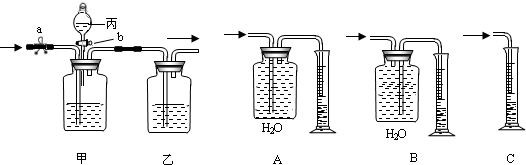
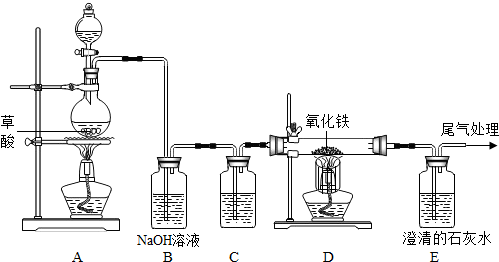
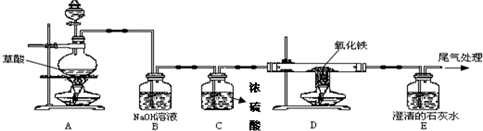
��֪���ᣨH
2C
2O
4���ڼ��������µķ�ӦΪH
2C
2O
4CO
2��+CO��+H
2O��������ͼ�ס���װ�ã�ͼ��a��bΪ�����Ŀ��أ������ɵ�CO��CO
2���з��벢����ɹ�ѡ�õ��Լ���ϡ���ᡢŨ���������������Һ��
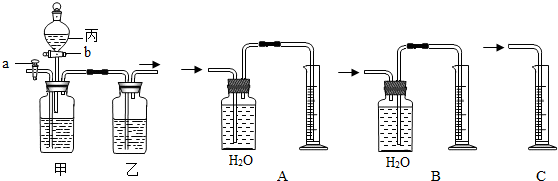
��1������a���رջ���b���û������ͨ���ס���װ�ã�����ʢ�ŵ��Լ�Ϊ
���йط�Ӧ�Ļ�ѧ����ʽΪ
����װ�������������
��
��2��Ҫ�������һ�����壬�����Ӧʢװ���Լ���
����ȷ�IJ���������
����ʱ��װ���з�����Ӧ�Ļ�ѧ����ʽΪ
��
��3����Ҫ�ⶨ���������CO���������������ͼA��B��C����װ����ѡ��һ������������װ��
��
��4������ʵ�����õ�CO��������Ϊ1232mL����ʵ��������CO���ܶ�Ϊ1.25g/L����ȫ��������ԭCuO�������Ͽ�����Cu�������Ƕ��ٿˣ�

 CO��+CO2��+H2O��������ͼ�ס���װ�ã�ͼ��a��bΪ�������أ����롢����ǰδ���������ɵ�CO��CO2���з��벢�����ѡ�õ��Լ���ϡ���ᡢŨ���������������Һ��
CO��+CO2��+H2O��������ͼ�ס���װ�ã�ͼ��a��bΪ�������أ����롢����ǰδ���������ɵ�CO��CO2���з��벢�����ѡ�õ��Լ���ϡ���ᡢŨ���������������Һ��