���û�ѧ����ʽ������������
��1��Сӱͬѧ��̽��������̼����ʱ����������̼����ͨ����ɫʯ����Һ��ʯ����Һ�ܿ���ɫ��ԭ����
H2O+CO2�TH2CO3
H2O+CO2�TH2CO3
�������������⣬��ҵ�ϳ���ϡ������������
Fe2O3+6HCl�T2FeCl3+3H2O
Fe2O3+6HCl�T2FeCl3+3H2O
��
��2�����ڴ��ʯ��ˮ�ĮJ������һ�㱡Ĥ���ñ�Ĥ�γɵ�ԭ����
CO2+Ca��OH��2�TCaCO3��+H2O
CO2+Ca��OH��2�TCaCO3��+H2O
����ʵ�����ﳣ��ϡ������ϴ��������Ӧ�Ļ�ѧ����ʽΪ
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
��
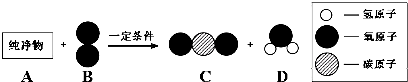
��3��������A����������ȼ�ϣ���һ��������A��B����ͼ��ʾ��ַ�Ӧ������C��D����֪A��һ�������к���3��̼ԭ�ӣ���A����Է�������Ϊ44����÷�Ӧ�Ļ�ѧ����ʽΪ
��




 ������������ϵ�д�
������������ϵ�д�
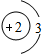 B��
B��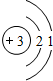 C��
C��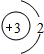 D��
D��
 22���ճ������ũҵ�����е��������ⶼ�뻯ѧ֪ʶ������أ�
22���ճ������ũҵ�����е��������ⶼ�뻯ѧ֪ʶ������أ�