



 ��ҵ����ϵ�д�
��ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
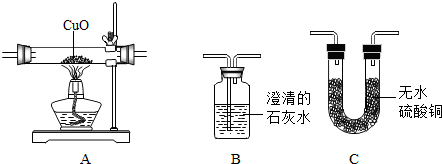
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
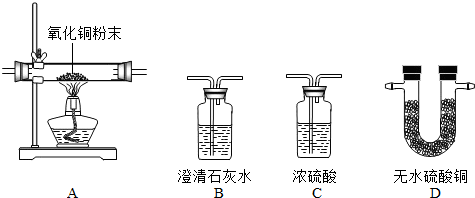
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
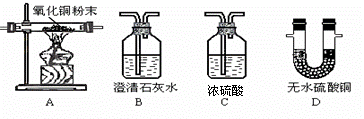 �κο�ѧ���۶�Ҫ����ʵ����֤��ʵ������Ҫ��ѧ��������ΪŪ���ij�����ķ��ն�������ɷ����Ƿ���ˮ������������һ����̼�Ͷ�����̼���ס�������ʵ��С��ֱ�������ʢ��ҩƷ��������ͼ�мг������Ⱦ�ʡ�ԣ���������ն�������ɷ֡�������B��C��D������ȫ��
�κο�ѧ���۶�Ҫ����ʵ����֤��ʵ������Ҫ��ѧ��������ΪŪ���ij�����ķ��ն�������ɷ����Ƿ���ˮ������������һ����̼�Ͷ�����̼���ס�������ʵ��С��ֱ�������ʢ��ҩƷ��������ͼ�мг������Ⱦ�ʡ�ԣ���������ն�������ɷ֡�������B��C��D������ȫ��
�ټ�����һ�����Ƿ�������ijһ�����壬���������Ƿ��ж�����̼�ķ���ʽ___________�������ն��ں���ˮ��������۲쵽��������_________����A�в������������ɺ�ɫ��ɺ�ɫ������ն��ڿ��ܺ���_________����Ҫȷ������һ����̼����Ҫ����__________(���ţ�����A��һ��������Ӧ�Ļ�ѧ����ʽ_______ ___________��
___________��
������������������װһ��װ�ã�ͨ��һ��ʵ��ͬʱ�����������壬������ͨ�����Ⱥ�˳�����ӵ����������ǣ����ţ����������ظ�ʹ�ã�__________����������ȫ�Ļ�����Ҫ���е�ʵ����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009���Ϻ��б�ɽ���п���ѧһģ�Ծ��������棩 ���ͣ������
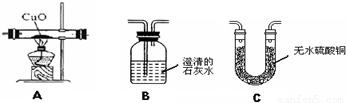
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com