��������������������Ƽ��ȷ����ձ�ʹ�ã�
��1������ij��ҵ��Ӫ���ƶ�ͣ����һ������������е�豸�����⼣�߰ߣ�����ͬѧ���豸��һö���������˿����������ϡ�����У��۲쵽��������ʧ���÷�Ӧ�Ļ�ѧ����ʽΪ
Fe2O3+6HCl�T2FeCl3+3H2O
Fe2O3+6HCl�T2FeCl3+3H2O
��һ��ʱ����ֹ۲쵽�������������ݲ�����д���������ݵĻ�ѧ����ʽ
Fe+2HCl�TFeCl2+H2��
Fe+2HCl�TFeCl2+H2��
�����ֽ��ѧ���Ļ�ѧ֪ʶ�������صĹ�����Ա�����һЩ��ֹ��е�豸��һ����ʴ�Ľ��飬��д��һ�����ֵĽ��飺
��������Ʒ���������������𰸾��ɣ�
��������Ʒ���������������𰸾��ɣ�
��
��2���ѣ�Ti���Ǻ��캽�ա������ȷ������Ҫ�������ϣ�����Ϊ��21���͵Ľ���������ҵ����þ�ڸ�������TiCl
4��Ӧ��ȡ����ѧ����ʽΪ��TiCl
4+2Mg
Ti+2MgCl
2���÷�Ӧ˵�������ѵĻ�Ա�þ
��
��
���ǿ������������
��3����ͭΪͭп�Ͻ���;��Ϊ�㷺��С��ͬѧ�Ӽ�������˻�ͭ��Ƭ������ͬѧ��һ�����������е�ͭ���������Ǿ����Ȳⶨ��ͭ���ᷴӦ�����������������ټ��㣮
��С������Ƴ�����ͼA�����ⶨ��
������ͭ��Ʒ����ƿ��ϡ�����������
��ͭ��Ʒ������ƿ������ϡ���ᣮ
����Ӧ��Ϻ��ٲ���ƿ�ͷ�Ӧ�����������������Ӧǰ����������Ϊ����������������
��С����С�����������Ľ����飺�����������е���ƿ�ϼ�һװ�и�����ĸ���ܣ��ⶨ��Ӧǰ��װ�õ����������˵��С�����иĽ��������ǣ�
��Ӧ��������ˮ�������������ݳ�
��Ӧ��������ˮ�������������ݳ�
��
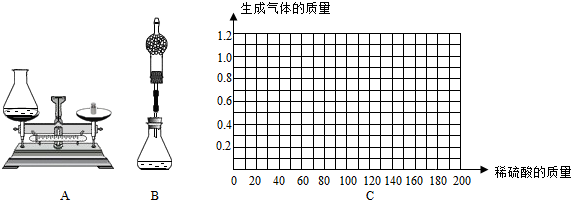
�ۻ���ͬѧΪ�˲ⶨ��ͭ��Ʒ��ɣ�ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼���±���
| ��Ʒ |
��1�� |
��2�� |
��3�� |
��4�� |
| ȡ��Ʒ������g�� |
50.0 |
50.0 |
50.0 |
50.0 |
| ȡϡ����������g�� |
40.0 |
80.0 |
120.0 |
160.0 |
| ��������������g�� |
0.4 |
0.8 |
1.0 |
1.0 |
�Լ��㣺
���������ڵ�1����Ʒ��õ������У�
����
����
�����������ƣ���ȫ��Ӧ�ˣ�
������ͼC�л�����50.0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼ��
���Լ����ͭм��Ʒ�е�п��������������д��������̣�����������һλС������