
��̼���������ǵ�����ϢϢ��أ�
��1�����������о�����̼Ԫ�أ�����Ӳ��������__________������ĸ��ţ���ͬ�����������������__________��

��2���ɱ��������������Ҫ������������__________�����������ѧ�������ʣ�
��3������̼�����ָ�����о������ٶ�����̼������������ŷţ����ֵ������������ġ��Ϳ�֧������������з��ϡ���̼�������__________������ĸ��ţ���
A���ճ�����У���ʱ�䲻�õ���ʱ��Ҫ�رյ�Դ
B������ʹ��˽�ҳ���������˹����������г�
C�������������ڲ����ò�ʱ���������ʹ��һ���Բ;ߣ�
��4��������ͼװ�ã���֤CO2�����ʣ�

�ټ��н�����ɫʯ����Һ��ֽ��������������__________��
�����г���ʯ��ˮ��������Ӧ�Ļ�ѧ����ʽΪ__________��
�۱����·�������Ϩ���Ϸ������Ϩ�𣬴�����֤��������̼����__________�����ʣ�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017������ʡ������μ�������꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ�������
��ȥ������̼�л��е�����һ����̼��Ӧ���õķ����ǣ� ��
A. ���������ͨ�����ȵĽ�̿ B. �ѻ�������ȼ
C. �ѻ������ͨ������ʯ��ˮ D. ���������ͨ�����ȵ�����ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ϻ������������꼶��ѧ����ĩ������⻯ѧ�Ծ��������棩 ���ͣ���Ϣ������
��ѧ���Լ������ԣ������ù���ͨ�õķ�������ʾ���ʵ���ɺͱ仯
��Ԫ�ط�����������Ļ�ѧ���ԡ����Ԫ�ط�����_______��
�ڻ�ѧʽ����Ԫ�ط��ű�ʾ������ɵ�ʽ�ӣ���N2��CO��SO2��HCl��Ca(OH)2�������γɵ�ˮ��Һ�ʼ��Ե���________�������������Ҫ������________������Ѫ�쵰��ϵ�����ʱ________��
�ۻ�ѧ����ʽ���ڱ�ʾ��ѧ��Ӧ��������ԭ����ͭ�Ļ�ѧ����ʽ��________��������������________��
�ܱ���(C3H8)��Һ��ʯ��������Ҫ�ɷ֣�C3H8��________��Ԫ����ɣ�0��25 mol C3H8��Լ����________��Hԭ�ӡ�C3H8ȼ�յĻ�ѧ����ʽ���£�����ƽ________
��C3H8+��O2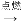 ��CO2+��H2O
��CO2+��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ϻ������������꼶��ѧ����ĩ������⻯ѧ�Ծ��������棩 ���ͣ�ѡ�������
������������ȼ�գ��������䡢���ɺ�ɫ������ǣ� ��
A. þ�� B. ľ̿ C. ���� D. ��˿
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ϻ������������꼶��ѧ����ĩ������⻯ѧ�Ծ��������棩 ���ͣ�ѡ�������
���������ѣ�TiO2���ɾ������ڿ�����TiO2��Ti�Ļ��ϼ�Ϊ�� ��
A. -4 B. -2 C. +2 D. +4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������ʡ�������ɽ�ؾ��꼶��ѧ����ĩ������⻯ѧ�Ծ��������棩 ���ͣ�ѡ�������
����ʵ�������ȷ����
A. �������������Ʒ�����ƽ���̵���ֽ�ϳ���
B. ϸ��˿��������ȼ��ʱ������ƿ��Ҫ��������ˮ����һ��ϸɳ
C. ʵ�������ʣ���ҩƷ����ֱ�ӷŻ�ԭ�Լ�ƿ��
D. �������ǵ�ľ�����뼯��ƿ�ڣ����������Ƿ��ռ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������ʡ�������ɽ�ؾ��꼶��ѧ����ĩ������⻯ѧ�Ծ��������棩 ���ͣ�ѡ�������
���������뻯ѧ����ʽ��ʾһ�µ���
A. ̼�ڿ����г��ȼ�� C+O2 CO
CO
B. ��˿�ڴ�������ȼ�� 4Fe+3O2 2Fe2O3
2Fe2O3
C. ����ȼ�� 4P+5O2 2P2O5
2P2O5
D. þ��ȼ�� Mg+O2 MgO2
MgO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ϻ��л�����2016-2017ѧ��Ⱦ��꼶��һѧ�����յ��в��Ի�ѧ�Ծ� ���ͣ�ѡ�������
ͬΪ1mol�����ʣ�����̼Ԫ������������
A. CH3COCH3 B. CH3COOH C. CH4 D. C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ϻ��г�����2016-2017ѧ����꼶��һѧ�ڽ�ѧ�������в��Ի�ѧ�Ծ� ���ͣ�ѡ�������
���������ࡱ�еġ�����ָ����
A. Ԫ�� B. ԭ�� C. ���� D. ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com