��2013?Ϋ���������仯����������������Ҫ�����ã���ش��������⣺
��1����ʢ�������ļ���ƿ�е�ȼϸ��˿��������ȼ�յĻ�ѧ����ʽ��
��Ϊ��ֹ����ƿ���ѣ�����ȡ�Ĵ�ʩ��
Ԥ���ڼ���ƿ�ײ���һЩˮ������ɳ��
Ԥ���ڼ���ƿ�ײ���һЩˮ������ɳ��
��
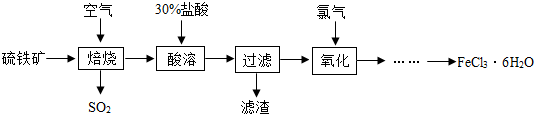
��2����֪�������Ȼ�����Ӧ�����Ȼ���������������������������Ҫ�ɷ���Fe
2O
3�����������У���ַ�Ӧ������ʣ�࣬д�������û���Ӧ�Ļ�ѧ����ʽ
Fe+2HCl=FeCl2+H2��
Fe+2HCl=FeCl2+H2��
����Һ�еĽ�����������
Fe2+
Fe2+
���÷��ű�ʾ����
��3����¯�����У���̿��������
���û�ѧ����ʽ��ʾ����
��4�������ۺ�ͭ�۵Ļ���������������Һ�У���Ӧ�������й���ʣ�࣮����˵����ȷ����
A
A
����д��ĸ��ţ���
A��ʣ�����϶�������
B��ʣ�����϶�������ͭ
C����Ӧ����Һ��һ����Fe
2+��Cu
2+D����Ӧ����Һ��һ����Ag
+��Cu
2+��



 ��У����ϵ�д�
��У����ϵ�д�