


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��ȤС��ͬѧΪ��̽��ʵ�����о��õ��������ƹ���ijɷ֣��������й�ʵ�飮����������һ���������̽�����
��ȤС��ͬѧΪ��̽��ʵ�����о��õ��������ƹ���ijɷ֣��������й�ʵ�飮����������һ���������̽�����| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��1��ȡ��������Ʒ�����Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ���������� | �� ��ɫ���� ��ɫ���� ���ɣ� |
˵��ԭ������һ���� Na2CO3 Na2CO3 �� |
| ��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ�� | ��Һ�� �� �� ɫ�� |
˵��ԭ������һ���� NaOH NaOH �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
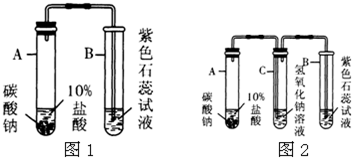
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
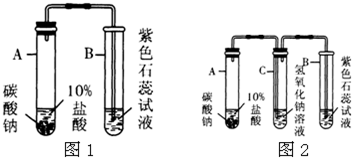
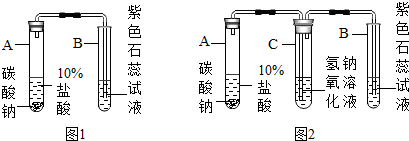
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007�긣��ʡ�������п���ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com