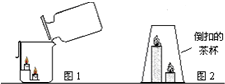
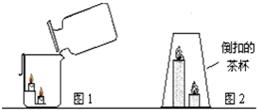
��2013?�����ģ�⣩��1��ij����С�����о�������̼������ʱ��������ͼ��ʾ����װ�ý������飮�ش��������⣮
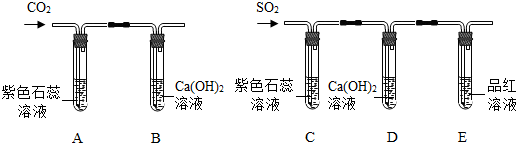
A�������
��Һ���
��Һ���
��
B�з�Ӧ�Ļ�ѧ����ʽ��
CO2+Ca��OH��2=CaCO3��+H2O
CO2+Ca��OH��2=CaCO3��+H2O
��
��2����С�����öԱȵķ����о�������������ʣ���������̽����
��������⡿���������������̼�����ڷǽ���������Ƿ�������ƵĻ�ѧ�����أ�
���������ϡ������������Ư���ԣ���ԭ���Ƕ���������ijЩ��ɫ���ʣ���Ʒ����Һ����Ӧ���ɲ��ȶ�����ɫ���ʣ�ʵ���ҳ��ú�ɫ��Ʒ����Һ�����������Ĵ��ڣ�
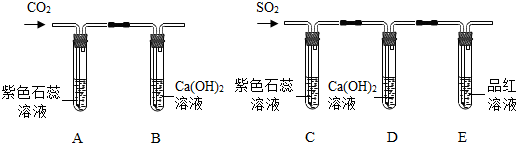
������̽��������װ����ͼ��ʾ��
��װ��C����ɫʯ����Һ��죬�����ڶ���������ˮ��Ӧ�����������ᣨH
2SO
3����װ��D�г���ʯ��ˮ����ǣ��÷�Ӧ�Ļ�ѧ����ʽ��
SO2+Ca��OH��2=CaSO3��+H2O
SO2+Ca��OH��2=CaSO3��+H2O
��
��װ��E���Թ�����Һ����ɫ��ȡ����Һ���ȣ���Һ�ֱ�Ϊ��ɫ����ԭ����
����������Ʒ����Һ��Ӧ���ɲ��ȶ�����ɫ���ʣ������ַֽ�������Ʒ��
����������Ʒ����Һ��Ӧ���ɲ��ȶ�����ɫ���ʣ������ַֽ�������Ʒ��
��
�۴�װ����һ�����ԵIJ���֮����
û�н���β������
û�н���β������
��
�ܻ���̿Ҳ��ʹƷ����Һ��ɫ������ɫԭ�����������ʹƷ����Һ��ɫ��ԭ������ͬ��������
����̿�����������������仯������������ʹ��Һ��ɫ�ǻ�ѧ�仯
����̿�����������������仯������������ʹ��Һ��ɫ�ǻ�ѧ�仯
����˼��ߡ�ijͬѧ����������ͨ�뵽��ɫ�����Ը��������Һ�У��۲쵽��Һ����ɫ��Ϊ��ɫ���ɴ����ó��Ľ����ǣ����������ܽ����Ը��������ҺƯ�ף������������֤���˽����Ƿ���ȷ��Ҫ��д�������������ۣ�
����ɫ�����Һ���ȣ��۲���Һ��ɫ�仯������Һ�ָ�Ϊ��ɫ����˽�������ȷ�ģ�����Һ���ָܻ�Ϊ��ɫ����˽��۲���ȷ
����ɫ�����Һ���ȣ��۲���Һ��ɫ�仯������Һ�ָ�Ϊ��ɫ����˽�������ȷ�ģ�����Һ���ָܻ�Ϊ��ɫ����˽��۲���ȷ
��
��������ۡ����������������̼�����ƵĻ�ѧ���ʣ�������������ijЩ����Ļ�ѧ���ʣ�



 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�